Figures & data
Figure 1. Study selection (PRISMA Flow chart)Citation30.
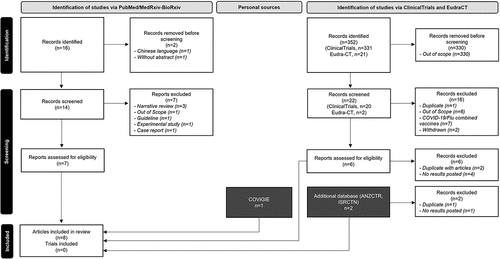
Figure 2. Study designs of selected randomized controlled trials (N = 3).
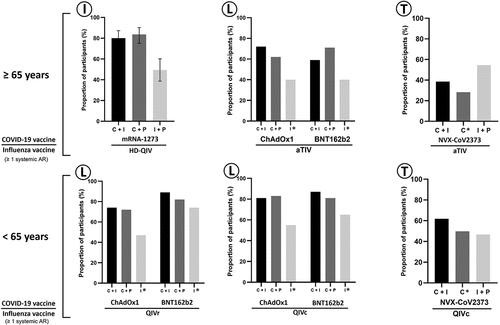
Table 1. Description of the safety & immunogenicity studies reviewed.
Figure 3. Proportion of vaccinees reporting at least one adverse reaction at injection site(s) through 7 d following mono or coadministration of influenza and COVID-19 vaccines.
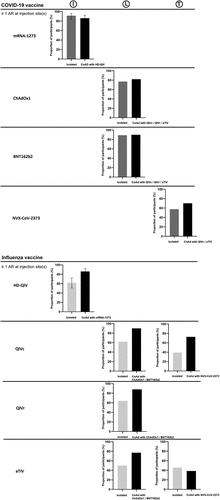
Figure 4. Proportion of vaccinees reporting at least one systemic adverse reaction through 7 d following mono or coadministration of influenza and COVID-19 vaccines.
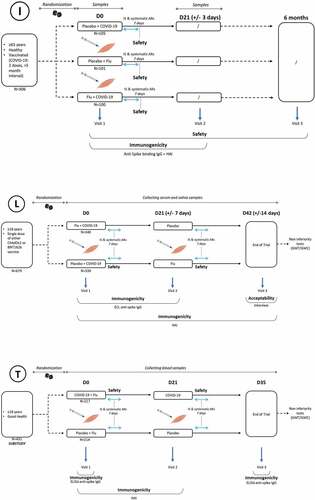
Table 2. Description of the acceptability & acceptance studies reviewed.
