Figures & data
Table 1. Demography and other baseline characteristics of all enrolled participants.
Table 2. Solicited local and systemic adverse events (AEs) reported in the 7 days after vaccine administration.
Figure 2. Geometric mean anti-Vi IgG antibody titers measured by ELISA (Group 1: Typbar-TCV® and MV co-administered at 9 months; Group 2: MV administered at 9 months and Typbar-TCV® at 10 months; Group 3: Typbar-TCV® administered at 8 months and MV at 9 months; and Group 4: only MV administered at 9 months. To assess TCV booster doses Group 1 was stratified into Group 1A, given a TCV booster at 10 months of age (28 ± 2 days after the first dose) and Group 1B, given a TCV booster at 15 months of age (180 ± 15 days post the first dose).
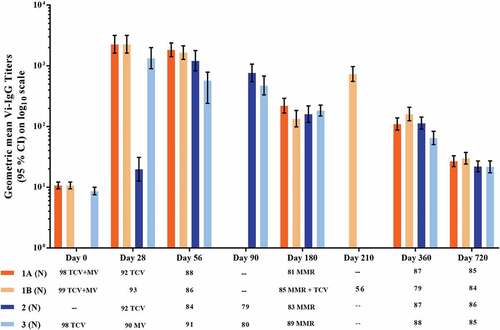
Table 3. Seroconversion rates for the anti-Vi IgG ELISA responses.
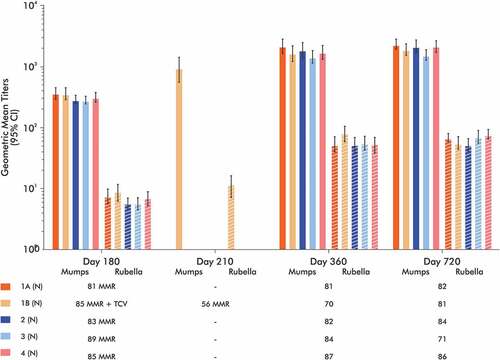
Table 4. Seroconversion rates of MMR vaccine antigens as measured by anti-measles, mumps and rubella IgG ELISA responses.
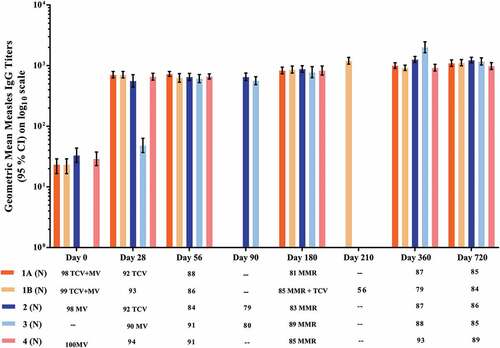
Figure 3. Geometric mean anti-measles IgG antibody titers measured by ELISA. All groups received MV at 9 months of age: concomitantly with Typbar-TCV® in Group 1; separately from Typbar-TCV® which was given at 10 months in Group 2; separately from Typbar-TCV® which was given at 8 months in Group 3; and with no Typbar-TCV® dose in Group 4. All groups received MMR at 15 months, which was administered concomitantly with Typbar-TCV® in Group 1B.