Figures & data
Table 1. Characteristics of adverse events following immunization (AEFI) with 9-valent HPV vaccine.
Figure 1. Reporting rate of adverse event following immunization of 9vhpv from 2019 to 2021, Zhejiang province (/10,000 doses).
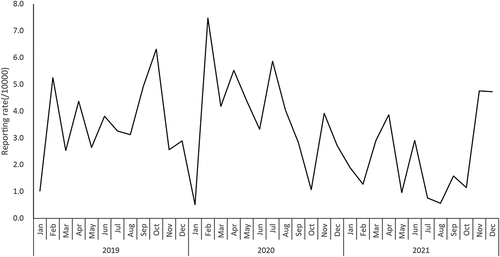
Table 2. Distribution of 9vHPV AEFI reports by city.
Table 3. Reporting rate of the 9vHPV AEFI by doses.
Table 4. Clinical diagnosis of AEFI reports following human papillomavirus 9-valent vaccine (9vHPV) from 2019–2021.
Table 5. Interval between AEFI onset and immunization.
