Figures & data
Figure 1. Flow chart of patient enrollment. Abbreviations: CHB, chronic hepatitis B; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; ICC, intrahepatic cholangiocarcinoma; IFN, interferon; NA, nucleoside/nucleotide analogues; w, weeks.
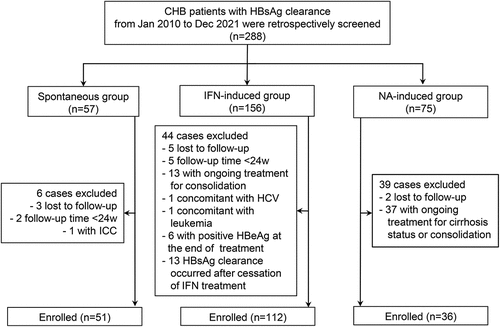
Table 1. Clinical characteristics of CHB patients with HBsAg clearance.
Figure 2. Cumulative incidences of HBsAg reversion according to the different HBsAg clearance methods. Abbreviations: HBsAg, hepatitis B surface antigen; IFN, interferon; NA, nucleoside/nucleotide analogues.
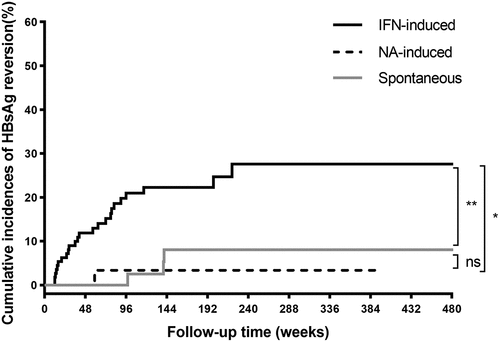
Figure 3. HBV vaccination in IFN-induced group. (a) The proportion of the patients with or without HBV vaccination. (b) The proportion of the patients with sequential administration vs. simultaneous administration of IFN and HBV vaccine. (c) The proportion of the patients receiving 1 dose, 2 doses or ≥ 3 doses of HBV vaccine. (d) The proportion of the patients with diverse HBsAb levels following HBV vaccination. (e) The proportion of responders or non-responders to HBV vaccination. (f) The proportion of the patients with weak or strong response to HBV vaccination. Abbreviations: HBV, hepatitis B virus; IFN, interferon.
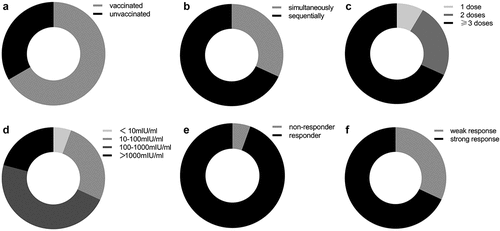
Table 2. Clinical characteristics of the patients with or without HBV vaccination in IFN-induced HBsAg clearance group.
Figure 4. Cumulative incidences of HBsAg reversion in IFN-induced group according to HBV vaccination. (a) The patients without HBV vaccination vs. with HBV vaccination. (b) The patients without HBV vaccination vs. responders to vaccination vs. non-responders to vaccination in the entire IFN-induced group. (c) The patients without HBV vaccination vs. with weak response to vaccination vs. with strong response to vaccination in the entire IFN-induced group. (d) The patients without HBV vaccination vs. with weak response to vaccination vs. with strong response to vaccination in individuals receiving sequential administration of IFN and HBV vaccine. Abbreviations: HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon.
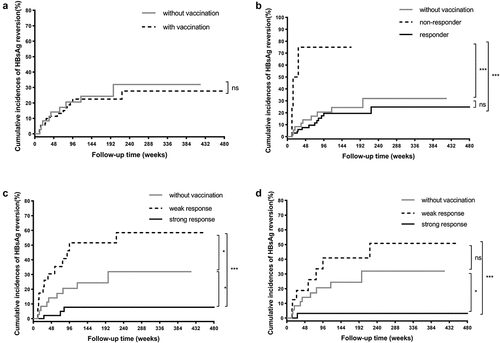
Table 3. Cox regression analysis of factors associated with the off-treatment HBsAg reversion in IFN-induced HBsAg clearance group.
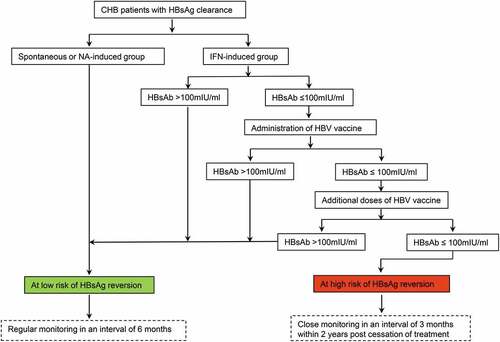
Figure 5. A new decision-making roadmap proposed to evaluate the risk of HBsAg reversion in CHB patients achieving HBsAg clearance by the different methods and to illustrate the application of HBV vaccine in reducing HBsAg reversion. Abbreviations: CHB, chronic hepatitis B; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon; NA, nucleoside/nucleotide analogues.