Figures & data
Figure 1. PRISMA flow diagram of study selection.
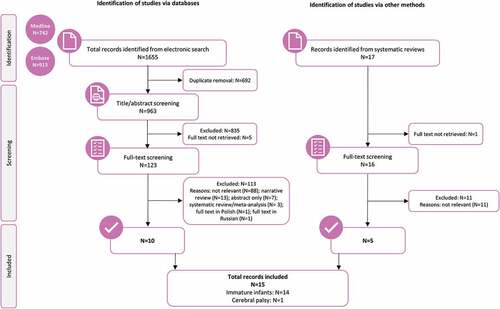
Table 1. Characteristics of studies included in the review.
Table 2. Seroconversion/Seropositivity rates after penta- or hexavalent vaccination.
Table 3. Antibody GMCs/GMTs after penta- or hexavalent vaccination and overall immunogenicity conclusion by the authors.
Table 4. Overall, serious, and cardiorespiratory adverse events after penta- and hexavalent vaccines.
Table 5. Compliance with penta- and hexavalent vaccine schedules.


