Figures & data
Figure 1. COVID-19 vaccines platforms. Types of various platforms used for the development of COVID-19 vaccines. Platforms based on viral components comprises protein-subunit vaccines which are isolated and purified viral proteins. Virus like particles (VLP) composed of viral proteins that mimic the structure of the virus, but no genetic material. DNA- and RNA-based vaccines are viral genetic material which encodes for viral proteins. Non-replicating viral vectors containing viral genetic material packaged inside viral vectors that cannot replicate. Replicating viral vectors containing viral genetic material packaged inside other viral vectors that can replicate. Whole virus-based vaccines are inactivated that contain copies of the virus that have been killed (inactivated) and live-attenuated which contains copies of the virus that have been weakened (attenuated).
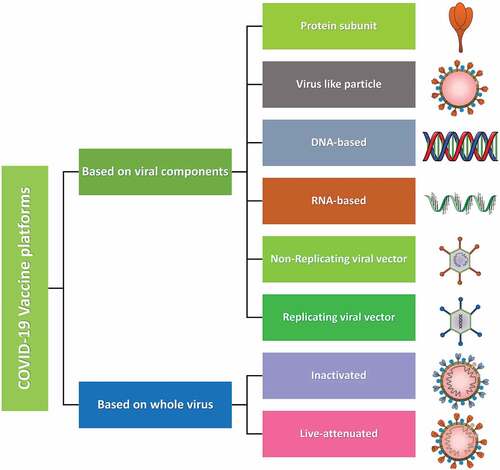
Figure 2. Status of COVID-19 vaccines. Bar graph showing the number of COVID-19 vaccines in different stages of clinical trials. Among the phase IV trials (n = 46), 17 are protein subunit vaccines, 2 are VLP vaccines, 1 is DNA vaccine, 8 are RNA vaccines, 7 are non-replicating viral vector vaccines, 1 is replicating viral vector, 9 are inactivated vaccines and 1 is live attenuated vaccine. In phase III trials (n = 46), 20 protein subunit vaccines, 1 is VLP vaccine, 2 are DNA vaccines, 12 are RNA vaccines, 3 are non-replicating viral vector vaccines, and 8 inactivated vaccines. In phase II trials (n = 72), 25 are protein subunit-based vaccines, 4 VLP based vaccines, 6 DNA based vaccines, 23 are RNA vaccines, 7 are non-replicating viral vector vaccines, 3 are replicating viral vectors, and 4 are inactivated vaccines. In phase I trial (n = 66), 18 are protein subunit vaccines, 2 are VLP vaccines, 6 are DNA based vaccines, 21 are RNA vaccines, 12 are non-replicating viral vector vaccines, 3 are replicating viral vector vaccines, and 4 are inactivated vaccines.
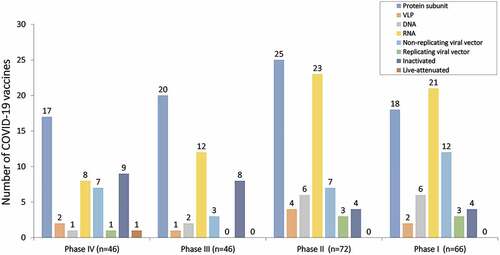
Table 1. Status of COVID-19 vaccines under phase III, and phase IV trials/approved.
Table 2. Status of COVID-19 vaccines under phase II trials/approved.
Table 3. Status of COVID-19 vaccines under phase I trials.
Table 4. Discontinued COVID-19 vaccines.
