Figures & data
Figure 1. Map of the recombinant plasmid pNMM vaccine and expression pattern of control plasmids.
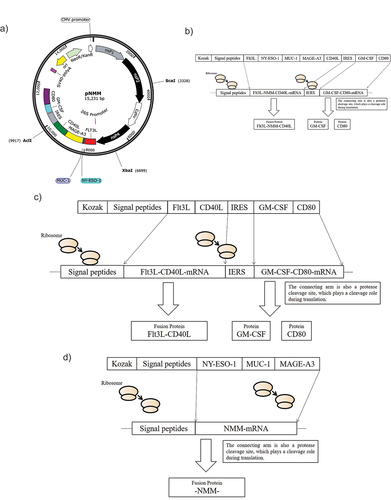
Figure 2. A schematic diagram of the experimental animal groups used in DNA vaccine immunogenicity experiments.
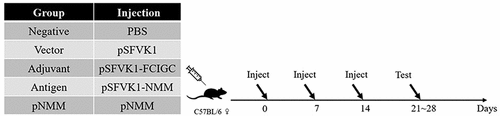
Figure 3. Expression of the pNMM vaccine in 293T cells.
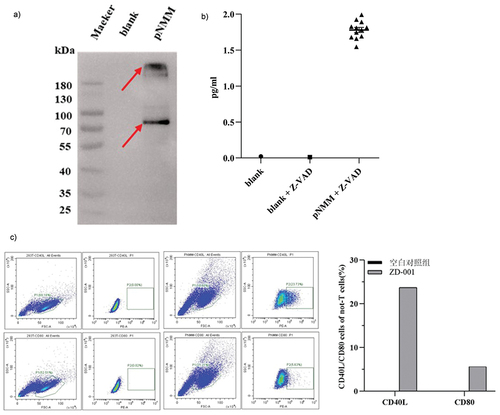
Figure 4. Evaluation of non-T cell response and specific IFN-γ-producing T cells.
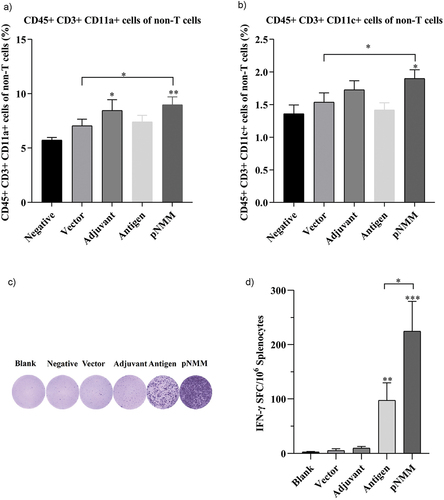
Figure 5. Protective efficacy of the DNA vaccine in a B16-NMM+-tumor model.
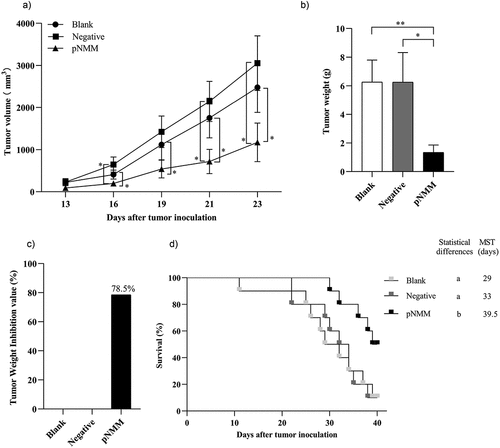
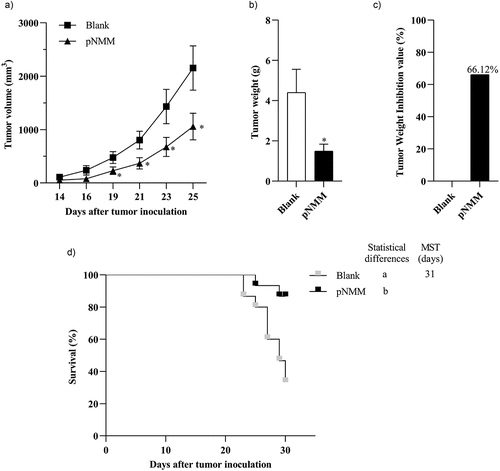
Figure 6. Therapeutic efficacy of the DNA vaccine in a B16-NMM+-tumor model.