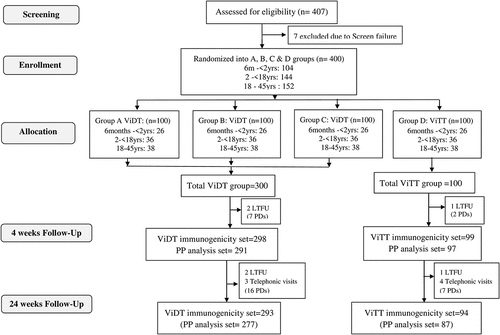
Figures & data
Table 1. Demographic distribution of the study participants (n = 400).
Table 2. Anti-Vi IgG seroconversion rates in Vi-DT vaccine vs Vi-TT vaccine at 4 weeks postvaccination: Immunogenicity set.
Table 3. Adverse events profile of participants after vaccination with Vi-DT vs Vi-TT vaccine.