Figures & data
Figure 1. Consort flow diagram. One hundred and sixty-five participants were screened and 150 determined eligible to participate in the study. Participants were randomized 1:1:1 into three study arms (50 each) and assessed pre-vaccination (baseline), four weeks after the first vaccination, two weeks after the second vaccination, and upon delivery (after excluding baseline seropositive patients).
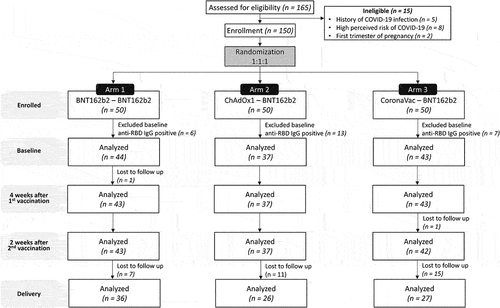
Table 1. Participant characteristics by COVID-19 vaccine regimen.
Figure 2. Maternal humoral immune responses four weeks after the first dose and two weeks after the second dose. (a) Illustrates anti-SARS-CoV-2 receptor binding domain (RBD) IgG geometric mean concentrations (GMCs), with references of the same vaccine regimens in healthy, non-pregnant adults for comparison.Citation22 (b) Illustrates geometric mean titers (GMTs) of neutralizing antibodies (NAbs) from 50% inhibitory dilution (ID50) by microneutralization assays against the ancestral Wuhan strain and Omicron BA.1, BA.2, and BA.5 subvariants. Titers below the lower limit of detection (LLOD of 1:10) were replaced with a value of 10. Error bars represent geometric means with 95% confidence intervals (CIs). Abbreviations: BAU, Binding Antibody Units; ID50, 50% Inhibitory Dilution.
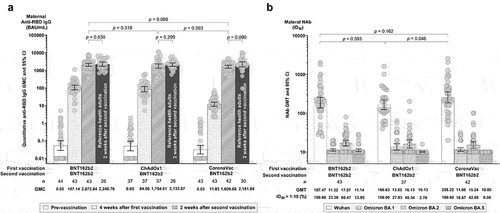
Figure 3. (a) Comparative geometric mean concentrations (GMCs) of maternal and cord blood anti-SARS-CoV-2 RBD IgG at delivery by vaccine regimen. (b) Pearson’s correlation coefficient (PCC) of umbilical cord anti-RBD IgG level against maternal anti-RBD IgG at delivery. (c) PCC of maternal anti-RBD IgG at time of delivery against time since the second vaccination. (d) PCC of umbilical cord blood anti-RBD IgG at the time of delivery against time since the second vaccination. (r) represents PCC.
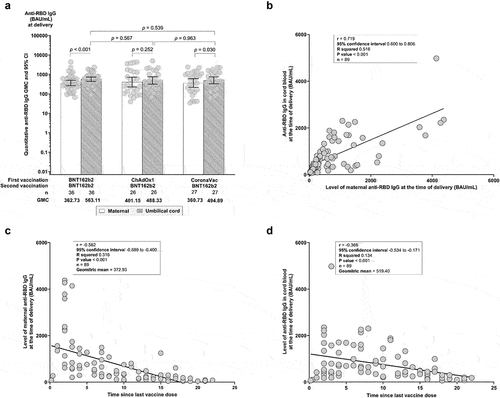
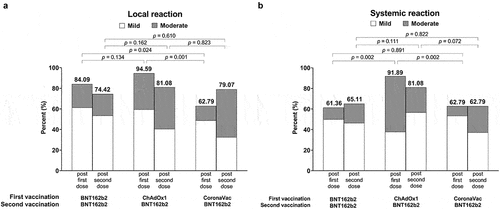
Figure 4. Self-reported local (a) and systemic (b) adverse events (AEs) within the first seven days after each vaccination by vaccine regimen.
Supplemental Material
Download PDF (268.2 KB)Data availability statement
Deidentified individual patient information and other datasets, study protocols, and documents generated and/or analyzed during this study will be made available after publication and shared to researchers with a methodologically sound proposal to achieve their aims upon request, by e-mail, to the corresponding author.
