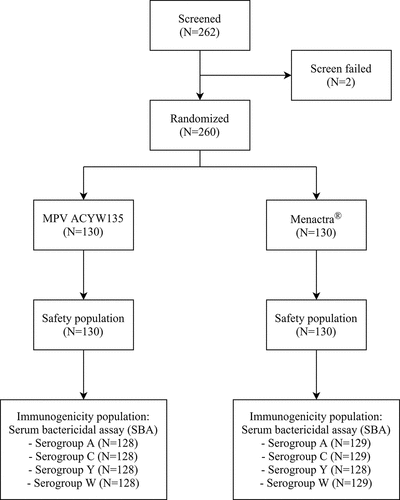
Figures & data
Table 1. Summary of Demographics at Baseline.
Table 2. Seroconversion rates as defined by proportion of subject with rSBA titers ≥128 at baseline and Day 30 post vaccination in Per-protocol analysis set (PP).
Table 3. Seroconversion rates as defined by proportion of subject with a rSBA ≥ 4-fold increase at Day 30 post vaccination as compared to baseline in per protocol analysis set (PP).
Table 4. Proportion of subjects with rSBA titers ≥8 at baseline and at Day 30 post vaccination in per protocol analysis set (PP).
Figure 2. Reverse Cumulative Distribution Curves of baseline rSBA titers - per-protocol analysis set (PP): blue curves stand for MPV ACYW135, and red curves stand forMenactra®.
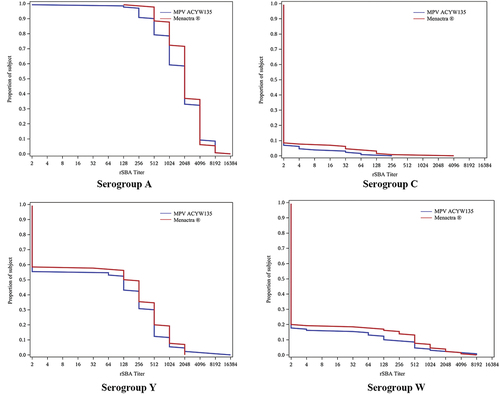
Figure 3. Reverse Cumulative Distribution Curves of rSBA titers 30 Days after Immunization - per-protocol analysis set (PP): blue curves stand for MPV ACYW135, and red curves stand for Menactra®.
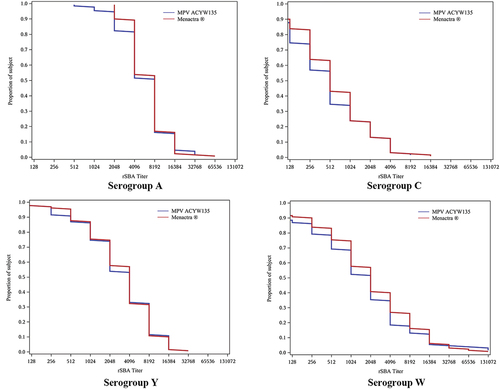
Table 5. Comparison of rSBA geometric mean titers between baseline and Day 30 post vaccination in per protocol analysis set (PP).
Table 6. Number (%) of subjects with solicited local and systemic reactions within 30 minutes to Day 7 post vaccination by severity.
Table 7. Number (%) of subjects with at least one unsolicited adverse events between Day 0 to Day 30 by severity.