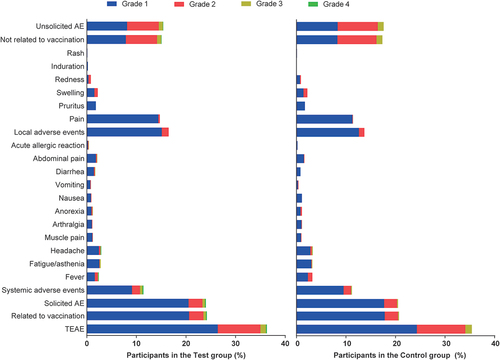
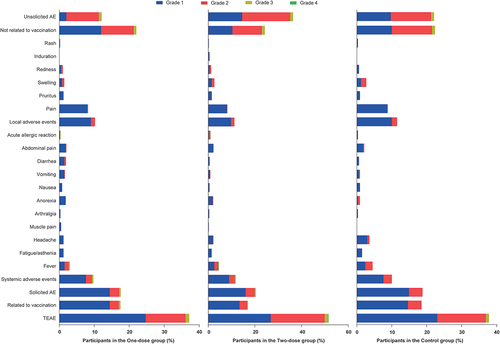
Figures & data
Figure 1. Participant disposition.
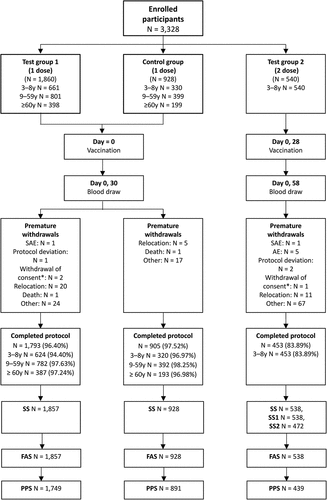
Table 1. Demographic data and baseline clinical characteristics of study participants (FAS).
Figure 2. Immunogenicity in all populations aged ≥3 years with pre-immunization antibody titers < 1:10 and pre-immunization antibody titers ≥ 1:10 (one-dose group) (FAS).
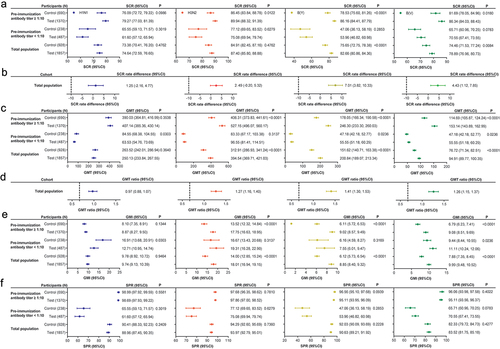
Table A1. List of dropouts.
Table A2. Demographic data and baseline clinical characteristics of participants by age group (FAS).
Table A3. Comparison of non-inferiority in various age groups between the investigational and control vaccines (one-dose group) (FAS).
Table A4. Immunogenicity in participants in various age groups with pre-immunization antibody titers < 1:10 and pre-immunization antibody titers ≥ 1:10 (one-dose group) (FAS).
Table A5. Immunogenicity in participants 3–8 years old (PPS).
Table A6. Immunogenicity in participants 3–8 years old with and without vaccination history in the one-dose group (PPS).
Table A7. Total adverse events after different immunization procedures in participants 3–8 years old.
Data availability statement
The protocol is saved at Dalian Aleph Biomedical Co., Ltd. and is available from the corresponding authors if necessary.