Figures & data
Figure 1. Study design. L denotes injection into the left leg, while R denotes injection into the right leg. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; e-diary = electronic diary; PCV13 = 13-valent pneumococcal conjugate vaccine.
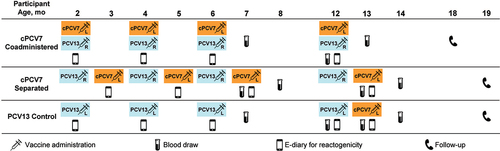
Figure 2. Participant disposition. Doses 1, 2, 3, and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. aIncludes participants who received their first dose of PCV13 but did not receive Dose 1 of cPCV7. bVaccinations and blood draws were terminated; however, safety follow-up procedures were continued per protocol. cA telephone contact was attempted for all participants who were 6 months beyond their last vaccination at the time of the data cutoff, unless consent was withdrawn. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine.
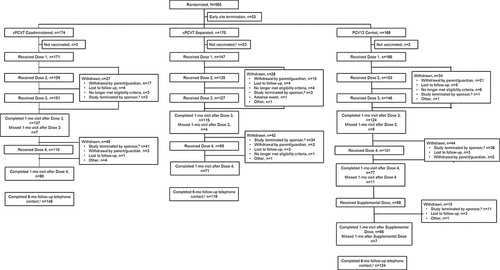
Table 1. Demographic Characteristics (Overall Safety Population).
Figure 3. Participants reporting (a) local reactions and (b) systemic events within 7 days after Dose 1, Dose 2, Dose 3, Dose 4, and Supplemental Dose (safety population). Doses 1, 2, 3, and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. For redness and swelling, mild was > 0.0 to 2.0 cm, moderate was > 2.0 to 7.0 cm, and severe was >7.0 cm. Pain at the injection site was mild if it hurt when gently touched (eg, participant whimpered, winced, protested, or withdrew), moderate if it hurt when gently touched with crying, and severe if it caused limitation of limb movement. For decreased appetite, mild was decreased interest in eating, moderate was decreased oral intake, and severe was refusal to feed. For drowsiness, mild was increased or prolonged sleeping bouts; moderate was slightly subdued, interfering with daily activity; and severe was disabling, not interested in usual daily activity. For irritability, mild was easily consolable; moderate was requiring increased attention; and severe was inconsolable, crying cannot be comforted. The numbers of subjects included in the analyses were as follows: Dose 1, n = 144‒166 per group; Dose 2, n = 126‒152 per group; Dose 3, n = 120‒143 per group; Dose 4, n = 79‒105 per group; Supplemental Dose, n = 85 for the PCV13 Control group. C = control; CA = coadministered; cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; S = separated.
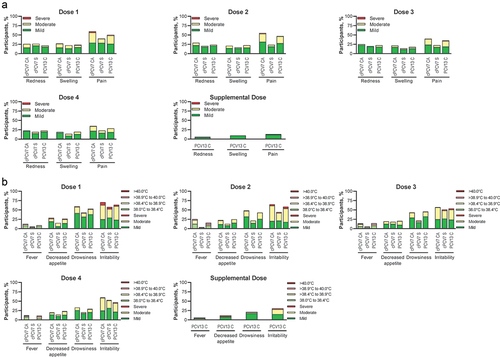
Table 2. Summary of AEs.
Figure 4. Serotype-specific (a) IgG GMCs and (b) percentages of participants achieving predefined IgG concentrations at 1 month after Dose 3 (cPCV7 serotypes) or 1 month after the third PCV13 dose (PCV13 serotypes; Dose 3 evaluable immunogenicity population). Dose 3 refers to the third dose of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or the third dose of PCV13 administered in the PCV13 Control group. For the PCV13 serotypes, data are shown from 1 month after the third dose of PCV13 for all groups. The predefined IgG concentration was ≥0.35 μg/mL for serotypes 1, 3, 4, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19F, 22F, 23F, and 33F; ≥0.23 μg/mL for serotype 5; ≥0.10 μg/mL for serotype 6B; and ≥0.12 μg/mL for serotype 19A.Citation18 Error bars display the upper and lower bounds of the 2-sided 95% CIs for (a) GMCs and (b) percentages of participants achieving predefined IgG concentrations, calculated based on the Student t distribution and the Clopper-Pearson method, respectively. Assay results below the LLOQ were set to 0.5 × LLOQ in the GMC calculations. The numbers of subjects included in the analyses were as follows: n = 128 for the cPCV7 Coadministered group, n = 94‒107 for the cPCV7 Separated group, and n = 109 for the PCV13 Control group. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMC = geometric mean concentration; IgG = immunoglobulin G; LLOQ = lower limit of quantitation; PCV13 = 13-valent pneumococcal conjugate vaccine.
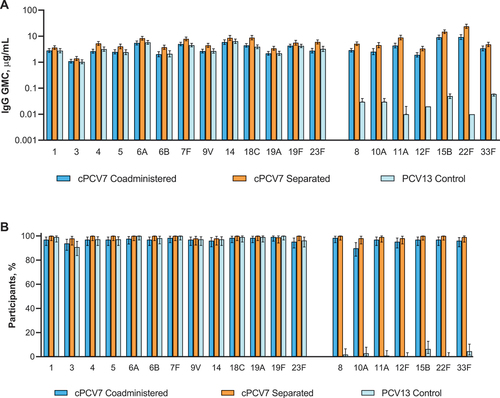
Figure 5. Serotype-specific OPA GMTs for the cPCV7 serotypes at (a) 1 month after Dose 3 (Dose 3 evaluable immunogenicity population), (b) 1 month after Dose 4 (Dose 4 evaluable immunogenicity population), and (c) 1 month after the Supplemental Dose (Supplemental Dose all-available immunogenicity population). Doses 3 and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. OPA titers were determined based on serum from a randomly selected subset of participants. Error bars display the upper and lower bounds of the 2-sided 95% CIs for GMTs calculated based on the Student t distribution. Assay results below the LLOQ were set to 0.5 × LLOQ in the GMT calculations. At 1 month after Dose 3, n = 38‒46 for the cPCV7 Coadministered group, n = 38‒45 for the cPCV7 Separated group, and n = 40‒44 for the PCV13 Control group. At 1 month after Dose 4, n = 24‒28 for the cPCV7 Coadministered group, n = 25‒28 for the cPCV7 Separated group, and n = 24‒29 for the PCV13 Control group. At 1 month after the Supplemental Dose, n = 21‒26 for the PCV13 Control group. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMT = geometric mean titer; LLOQ = lower limit of quantitation; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine.
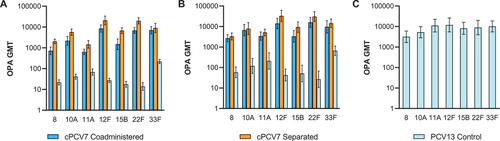
Figure 6. Serotype-specific IgG GMCs 1 month after Dose 4 (cPCV7 serotypes) or 1 month after the fourth PCV13 dose (PCV13 serotypes; Dose 4 evaluable immunogenicity population) and 1 month after the Supplemental Dose (Supplemental Dose all-available immunogenicity population). Dose 4 refers to the fourth dose of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or the fourth dose of PCV13 administered in the PCV13 Control group. For the PCV13 serotypes, data are shown from 1 month after the fourth dose of PCV13 for all groups. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. Error bars display the upper and lower bounds of the 2-sided 95% CIs for GMCs calculated based on the Student t distribution. Assay results below the LLOQ were set to 0.5 × LLOQ in the GMC calculations. At 1 month after Dose 4, n = 76 for the cPCV7 Coadministered group, n = 52‒57 for the cPCV7 Separated group, and n = 68 for the PCV13 Control group. At 1 month after the Supplemental Dose, n = 66 for the PCV13 Control group. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMC = geometric mean concentration; IgG = immunoglobulin G; LLOQ = lower limit of quantitation; PCV13 = 13-valent pneumococcal conjugate vaccine.
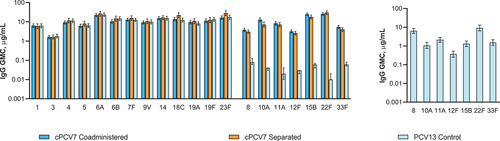
Table 3. IgG GMFRs for the cPCV7 Serotypes from Before Dose 4 to 1 Month After Dose 4a (Evaluable Immunogenicity Population) and from Before Supplemental Dose to 1 Month After Supplemental Dosea (PCV13 Control Group Only; Supplemental Dose All-Available Immunogenicity Population).
Table 4. OPA GMFRs for the cPCV7 Serotypes from 1 Month After Dose 3a to 1 Month After Dose 4a (Evaluable Immunogenicity Population) and from Before Supplemental Dose to 1 Month After Supplemental Dosea (PCV13 Control Group Only; Supplemental Dose All-Available Immunogenicity Population)b.
Data availability statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
