Figures & data
Figure 1. Genetic structure of the four TAK-003 vaccine strains. Abbreviations: C, capsid; E, envelope; NS, nonstructural protein; prM, premembrane; TDV-1/2/3/4, dengue serotype 1/2/3/4 strain. Reproduced with permission from Patel et al.Citation42.
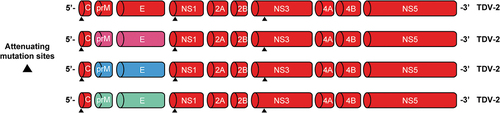
Figure 2. Trial visits schematic. Abbreviations: MAAEs, medically attended adverse events; SAEs, serious adverse events. Safety assessments were as follows: solicited local events (recorded up to 30 minutes, and up to 7 days after each TAK-003 dose), solicited systemic events (recorded up to 30 minutes, and up to 14 days after each TAK-003 dose), unsolicited events (recorded up to 28 days after each TAK-003 dose), and MAAEs and SAEs recorded for the entire duration of trial. Solicited local AEs comprised injection site pain, injection site erythema, and injection site swelling. Solicited systemic AEs comprised asthenia, headache, malaise, myalgia, and fever. MAAEs were defined as AEs leading to an unscheduled visit to, or by, a healthcare professional including visits to an emergency department, but not fulfilling seriousness criteria.
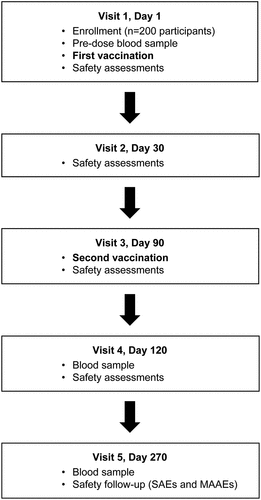
Figure 3. (a) Participant allocation to safety, full analysis and per-protocol sets. *Any participant not included in the per-protocol set if he/she experienced one or more trial protocol deviation(s) and (b) Participant disposition for the DEN-307 clinical trial. Abbreviations: AE, adverse event; LTF, lost to follow up; WOC, withdrawal of consent.
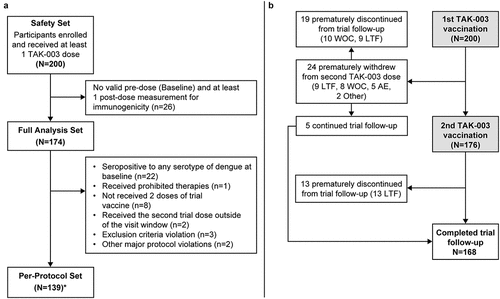
Table 1. Participant demographic and baseline characteristics.
Figure 4. (a) Percentage (95% CI) of participants who were seropositive against each DENV serotype at days 120 and 270 and (b) Percentage (95% CI) of trivalent and tetravalent baseline seropositive participants (per-protocol set). Baseline seropositive defined as a reciprocal neutralizing antibody titer ≥ 10 to 1 dengue virus serotypes. Baseline seronegative defined as titer < 10 to all dengue serotypes. Abbreviations: CI, confidence interval; DENV, dengue virus.
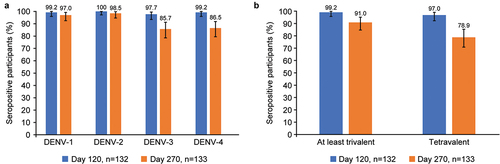
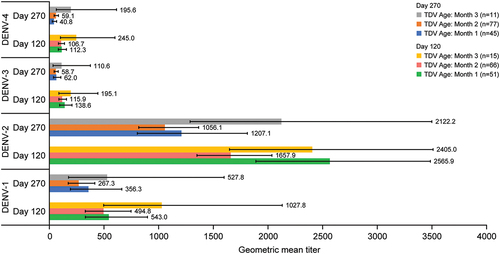
Figure 5. Geometric mean titers (95% confidence interval) of neutralizing antibodies measured by MNT50 for each dengue serotype (per-protocol set). Abbreviations: DENV, dengue virus; MNT50, microneutralization test 50%.
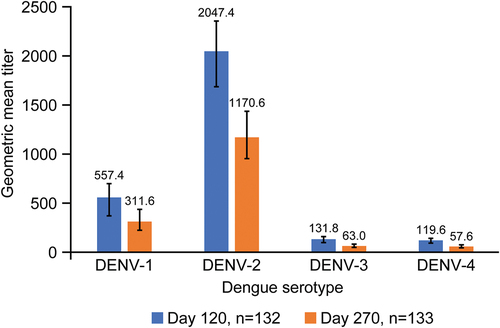
Table 2. TAK-003 overview of adverse events.
Table 3. Summary of solicited local adverse events within 7 days post vaccination (safety set).
Table 4. Summary of solicited systemic adverse events within 14 days post vaccination (safety set).
Table 5. Summary of unsolicited adverse events up to 28 days post vaccination, by preferred term (safety set).
Table 6. Summary of medically attended adverse events (MAAEs) post vaccination up to the end of study (safety set).
Figure 6. Baseline dengue seronegative participant geometric mean titers (95% confidence interval) at day 120 and day 270 after TAK-003 administration in trials DEN-301,Citation19,Citation20,Citation29 DEN-304Citation25 and DEN-307. Abbreviation: DENV, dengue virus.
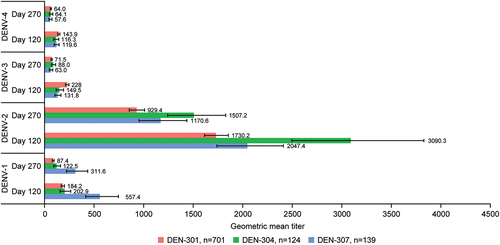
Figure 7. Baseline dengue seronegative participant geometric mean titers (95% confidence interval) at day 120 and day 270 following administration of TAK-003 in trial DEN-307. Abbreviation: DENV, dengue virus.