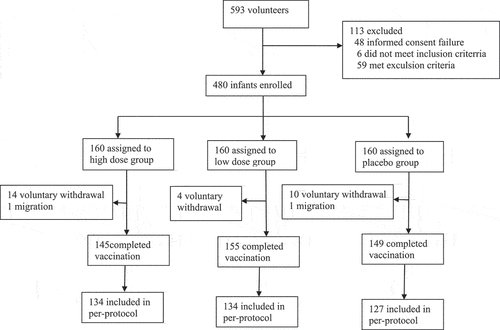
Figures & data
Table 1. Summary of demographic characteristics.
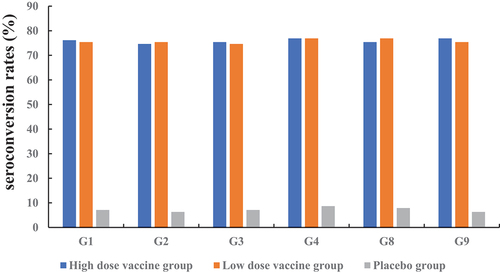
Table 2. Summary of IgA response to serotypes G1, G2, G3, G4, G8, and G9 in the per-protocol immunogenicity population.
Table 3. Summary of adverse events (AEs).
Table 4. Summary of solicited adverse events (AEs).