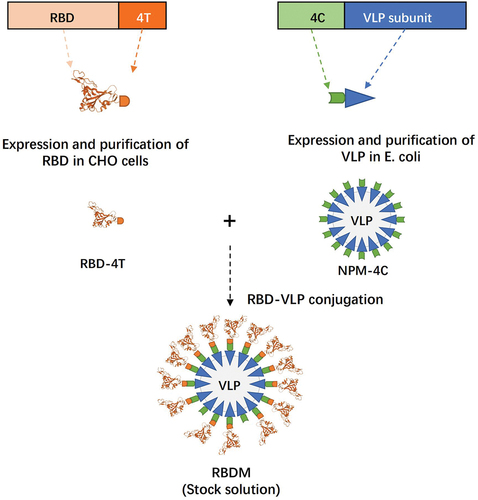
Figures & data
Figure 2. Study profile.
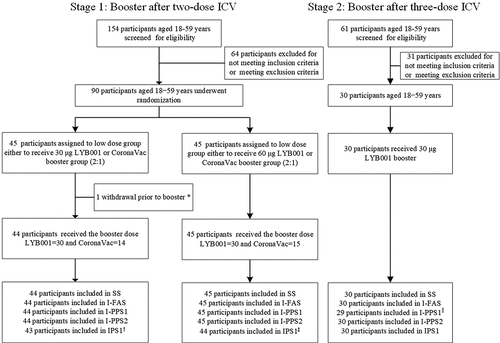
Table 1. Baseline characteristics of the participants.
Table 2. Overall adverse events or reactions after booster administration.
Figure 3. VSV-based neutralizing antibody titers against prototype SARS-CoV-2 and Omicron BA.4/5 strain.
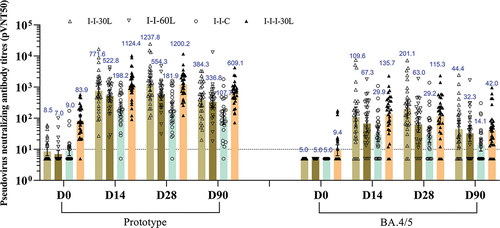
Figure 4. The spike protein binding IgGs at baseline and 14, 28, and 90 days after booster administration.
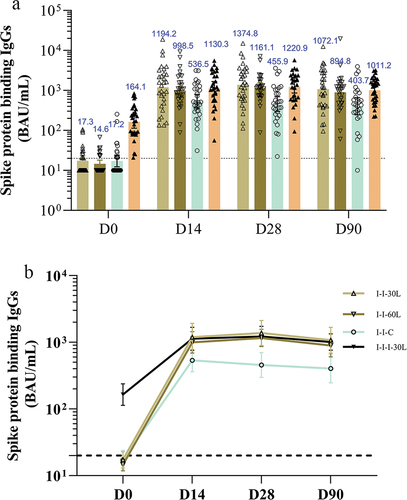
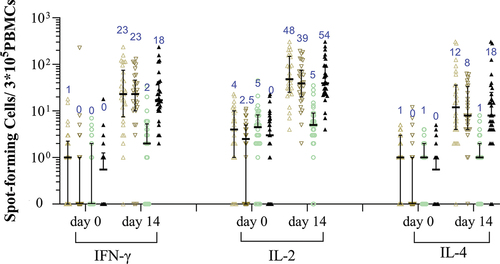
Figure 5. RBD-specific IFN-γ, IL-2, or IL-4 secreting T-cells measured by ELISpot assay.
Supplemental Material
Download PDF (236.7 KB)Data availability statement
The data used in this study are available from the corresponding author upon reasonable request.