Figures & data
Figure 1. Participant flow from visits 1–8 for all groups.
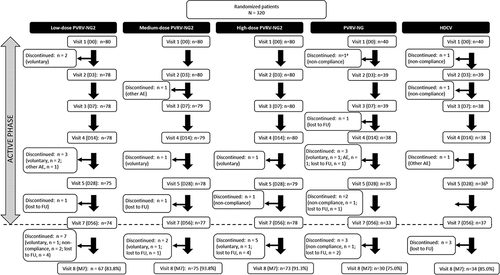
Table 1. Baseline characteristics for the full analysis set (N = 320).
Figure 2. Post-vaccination geometric mean rabies virus neutralizing antibody (RVNA), determined by RFFIT (modified full analysis set).
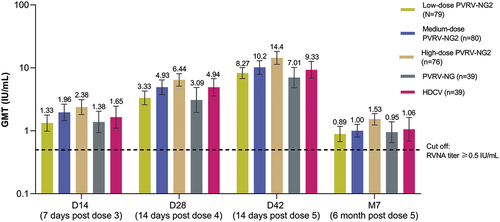
Table 2. Immunogenicity summary of RVNA titers and GMTs on D14, 28, and 42 (per protocol analysis set).
Table 3. Ratio of geometric mean titer (GMT) between groups on D14, 28, and 42 (modified full analysis set).
Figure 3. Post-vaccination: rabies virus neutralizing antibody (RVNA) titers determined by RFFIT≥0.5 IU/mL (per-protocol analysis set).
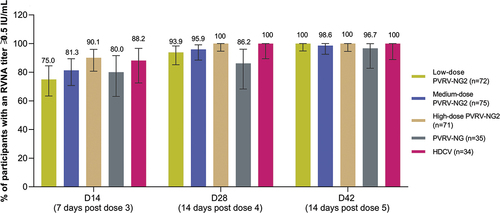
Table 4. Immunogenicity summary of RVNA titers 6 months after the final injection in participants with a baseline titer <0.5 IU/mL (modified full analysis set).
Table 5. Number of patients with determined virus neutralization results and achieving complete neutralization at 1:5 dilution in RFFIT (per-protocol analysis set).
Table 6. Safety profile of low-, medium-, and high-doses of PVRV-NG2, PVRV-NG, or HDCV for solicited and unsolicited reactions (safety analysis set).
Data availability statement
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of study participants. Further details on Sanofi’s data-sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
