Figures & data
Table 1. Subject disposition.
Table 2. Baseline characteristics of study participants.
Table 3. HPV 9 Administration information.
Table 4. The summary of AE.
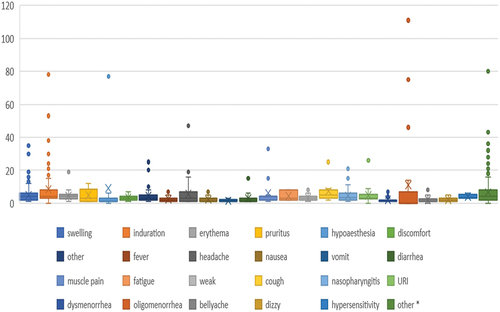
Table 5. AEs occurred within 30 days following any HPV9 vaccination.
Table 6. Non-vaccination-site AEs related to HPV 9 within 30 days.
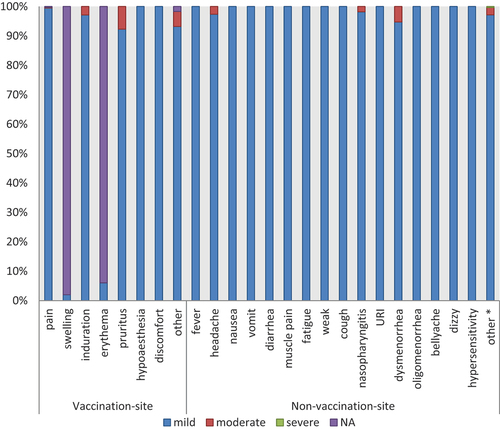
Table 7. Grade of adverse events after HPV9 vaccination.