Figures & data
Figure 1. Schematic diagram of T-cell epitope distribution in nDnak and DnaK. The scale unit is amino acid (AA).
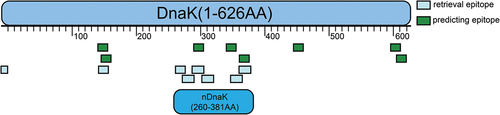
Figure 2. Construction of rBCG-ECD003. (a) Schematic of the mycobacteria-E. coli shuttle vector pMV361-ECD003. (b) Agarose gel electrophoresis of the PCR products amplified from rBCG-ECD003, transformed with pMV361 shuttle vectors. Lane 1, DNA marker; lane 2, the PCR product amplified from rBCG-pMV361; lane 3, the PCR product amplified from rBCG-ECD003.
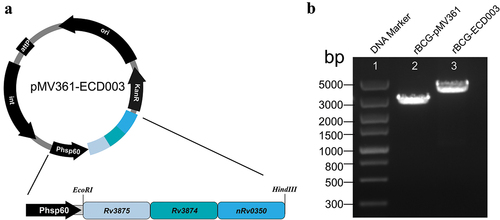
Figure 3. Superior cytokines production profile induction in the spleen lymphocytes of the BALB/c mice immunized with rBCG-ECD003. The cytokines production profile was analyzed in isolated spleen lymphocytes at the 4th and 12th week after immunization. 1 × 105 cells were restimulated with the fusion protein ECD003 (a) or PPD (b) and the concentration of cytokines (IFN-γ, TNF-α, IL-2, IL-12, IL-4, IL-6, IL-10, IL-17 and GM-CSF) was analyzed with multiplex cytokine assay. Results were shown as the mean ± SEM (n = 3) per group. Statistical significance was determined by ANOVA with Tukey’s multiple comparisons tests (*p < .05, **p < .01).
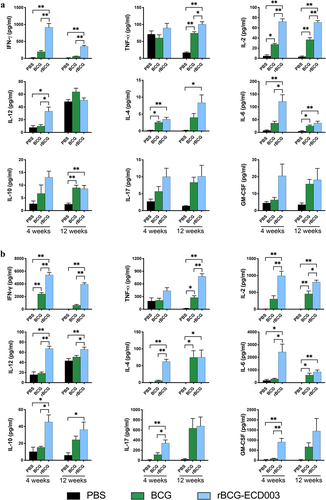
Figure 4. Vaccination with rBCG-ECD003 enhanced long-term responses of polyfunctional CD4+T cells in the spleen lymphocytes. Intracellular cytokine staining was performed in isolated spleen lymphocytes at the 12th week post-vaccination. The proportion of cytokine-producing CD4+T cells after stimulation with the ECD003 (a) and PPD (b) respectively were shown as the mean ± SEM (n = 3) per group. Pie charts reflected the average proportion of ECD003-responding (c) and PPD-responding (d) CD4+T cells with each combination of cytokine production. Statistical significance was determined by ANOVA with Tukey’s multiple comparisons tests (*p < .05, **p < .01).
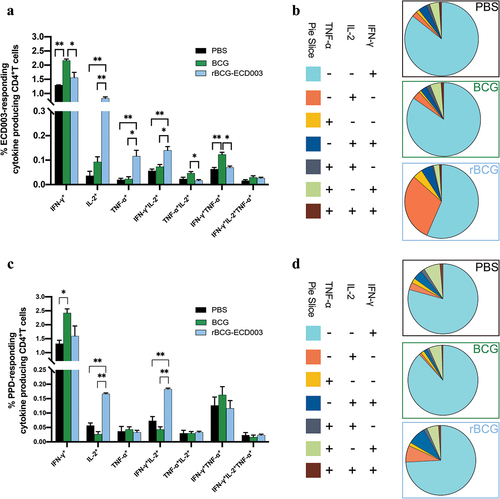
Figure 5. rBCG-ECD003 conferred superior humoral immunity in BALB/c mice. Assay of IgG antibodies against ECD003 (a) and PPD (b) in the sera of the immunized BALB/c mice. Serum samples were collected at the 4th, 8th, and 12th week after immunization, and the titers of IgG were detected by ELISA. The results were shown as the Log2 of the mean ± SEM (n = 6) per group. Statistical significance was determined by Student’s t-test. (*p < .05, **p < .01).
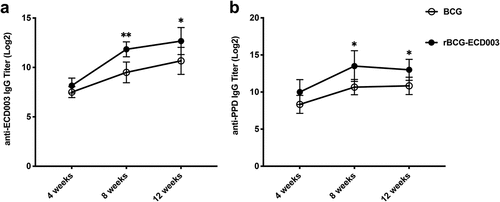
Figure 6. The protective efficacy of rBCG-ECD003 in vitro. The BALB/c mice (n = 6) were s.C. immunized with PBS, BCG, and rBCG-ECD003 respectively. The spleen lymphocytes were isolated 4th and 12th week post-immunization and co-cultured with ~ 500 CFU of mtb H37Rv. The bacterial load results of the 4th week (a) and 12th week (b) were presented as mean ± SEM. Statistical significance was determined by ANOVA with Tukey’s multiple comparisons tests (*p < .05, **p < .01).
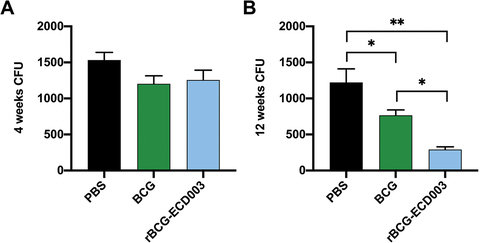
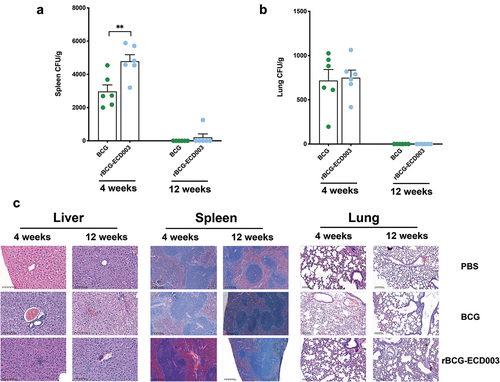
Figure 7. The safety analysis of the rBCG-ECD003 vaccine after immunization. The BALB/c mice were immunized with 1 × 106 CFU of the parental BCG or rBCG-ECD003. At the 4th and 12th week, the mice were sacrificed, and the parental BCG and rBCG-ECD003 CFU recovered from lung (a), and spleens (b) The liver, spleen, and lung samples were collected and performed with H&E staining (c). The typical pathological changes in each group were shown.