Figures & data
Figure 1. CONSORT flow diagram showing the number of patients who were screened, randomized into the treatment groups, completed the study, and included in the analysis.
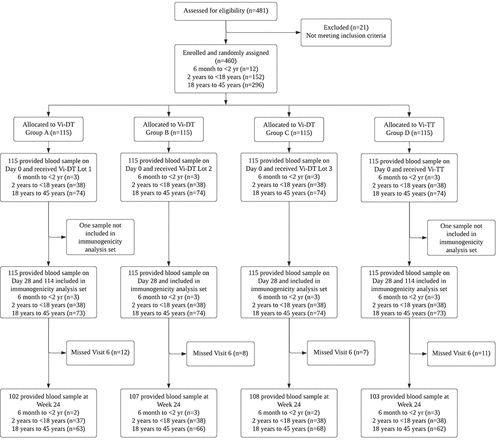
Table 1. Demographics information of enrolled participants.
Table 2. Anti-vi-IgG ELISA response for seroconversion - non-inferiority data at week 4 for immunogenicity set.
Table 3. Anti-vi-IgG ELISA GMT at week 24 for immunogenicity set.
Table 4. Solicited adverse Events.
Table 5. Distribution of solicited adverse events.
Table 6. Unsolicited adverse events.
Table A1. GMT of anti-vi IgG ELISA response at week 4 for Dhulikhel Hospital – immunogenicity set.
Table A2. Seroconversion rate of anti-vi IgG ELISA response at week 24 for Dhulikhel Hospital – immunogenicity set.
