Figures & data
Figure 1. Disposition of participants in Safety Set and per protocol immunogenicity sets of the three study phases.
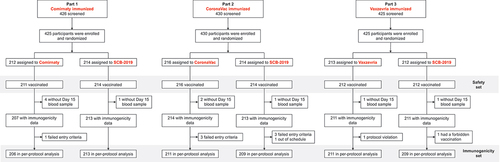
Table 1. Demographics of the exposed study populations (Safety Set).
Table 2. Viral neutralizing antibody (VNA) titers expressed as international units per mL (95% CI) at Day 15 in the study groups (Per Protocol Sets).
Figure 2. Anti-prototype SARS-CoV-2 neutralizing GMTs (IU/mL) with 95% CI at Days 1 and 15 in the three parts of the study according to baseline immunity (Per Protocol Set). Participants are grouped as low (25% with lowest titers), medium (50% with medium titers) and high (25% with highest titers) baseline. Heterologous to homologous GMT ratios (95% CI) at Day 15 are shown above columns; values at bases of columns show numbers of participants per group.
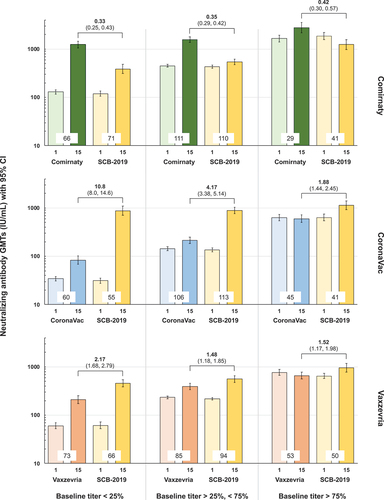
Figure 3. neutralizing GMTs (95% CI) against prototype SARS-CoV-2 and variants at Days 1 and 15 in subsets from the three parts of the study (Omicron BF.7, BQ.1.1.3 and XBB1.5 were not tested in the CoronaVac cohort).
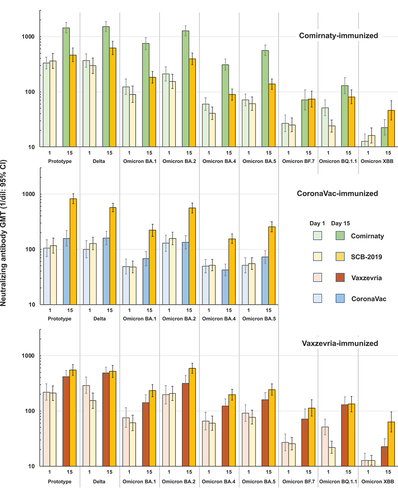
Table 3. Geometric mean-fold rises (95% CI) of neutralizing antibodies against prototype and SARS-CoV-2 variants from Day 1 to Day 15.
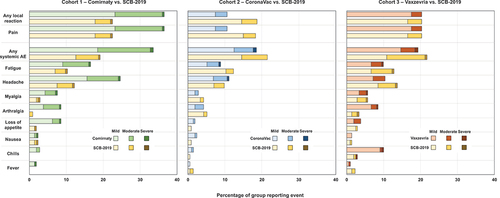
Table 4. Incidences of solicited and unsolicited adverse events, SAEs, MAAEs and AESIs (Safety Set).