Figures & data
Figure 1. Flowchart describing cases and controls included in the study.
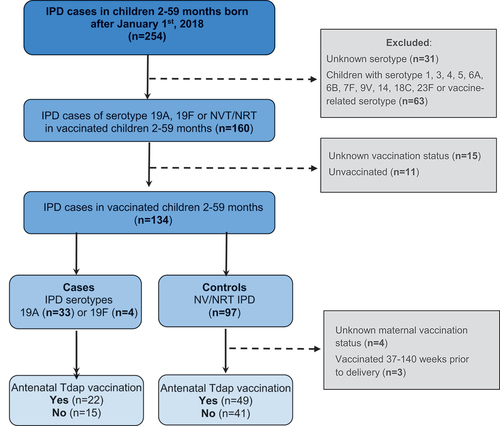
Table 1. Summary of sociodemographic variables collected for all IPD patients.
Data availability statement
Due to the nature of the research and to ethnical and legal restrictions, supporting data is not available.
