Figures & data
Figure 1. Schematic representation of the T-DNA region of the pBYR2e RSV-F Fc fusion plant expression vector. The T-DNA region plays a crucial role in facilitating the transfer of the gene of interest into plant cells. It includes the left border (LB) and right border (RB) which serve as the boundaries for gene transfer. The Pin II 3’ sequence derived from potato proteinase inhibitor II acts as a border element helping to facilitate the insertion of the desired genes into the plant genome. The vector also incorporates several important components, such as the Tomato Bushy Stunt Virus (TBSV) RNA silencing suppressor, P19; the Cauliflower Mosaic Virus (CaMV) 35s promoter, P35s; the CaMV enhancer, P35s×2; the tobacco extension gene region, Ext3’ FL, 3‘; the tobacco RB7 promoter, Rb7 5’ del; the Bean Yellow Dwarf Virus (BeYDV) short intergenic region, SIR; the BeYDV long intergenic region, LIR; and the BeYDV replication initiation proteins, Rep and RepA, along with C2/C1.Citation41.
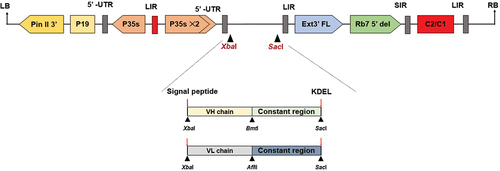
Figure 2. SDS–PAGE (a) and Western blot (b) for purified plant-produced anti-RSV monoclonal antibodies (mAbs) under nonreducing conditions. The Western blot (b) was probed using HRP-conjugated anti-human gamma chain antibody. For reducing conditions, the membrane was probed with either HRP-conjugated anti-human gamma chain antibody (c) or anti-human kappa chain antibody (d). Lane M, protein ladder; lane 1, purified plant-produced 5C4; lane 2, purified plant-produced CR9501.
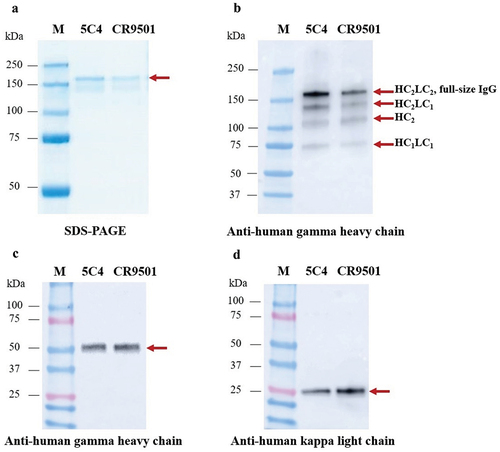
Figure 3. Size exclusion analysis of purified plant-produced anti-RSV mAbs. Purified mAbs were separated in the XBridge protein BEH 200 column. A total of 5 µg of purified mAb was loaded on the column and run at 0.3 mL/min for 20 minutes, UV detection at 280 nm, and the column temperature was maintained at 25°C.
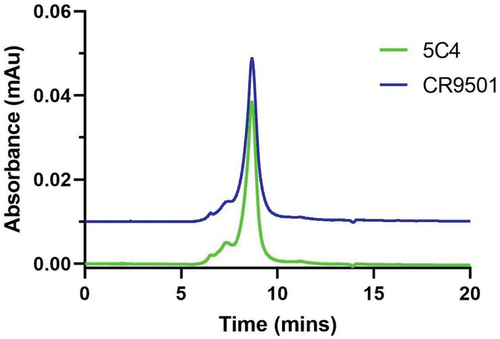
Table 1. Purity of plant-produced anti-RSV antibodies using SEC-UHPLC.
Figure 4. Liquid chromatography mass spectrometry (LC/MS) analysis illustrating the N-glycosylation profiles of the heavy chain peptide EEQYNSTYR (mass: 1189.51 Da) from plant-produced anti-RSV monoclonal antibodies. The peaks specifically assigned to mannose N-glycans (Man5-Man9) are illustrated, providing insights into the glycan composition associated with the analysed glycopeptide. corresponds to the 5C4 antibody, while represents the CR9501 antibody.
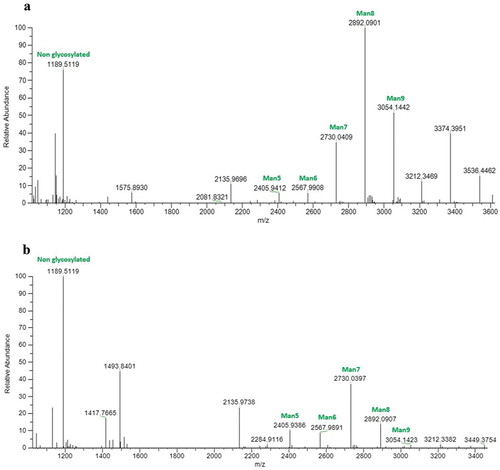
Figure 5. Binding characteristics of plant-produced anti-RSV mAbs. ELISA was used to quantify the specific binding to the RSV-F his-tagged monomer protein. The purified plant produced anti-RSV mAbs, and the plant produced nivolumab (anti PD-1, as a negative control). The binding activity of the antibody was detected with HRP-conjugated anti-human IgG antibody. The data are shown as the mean of triplicates and error bars represent standard deviation. The EC50 was calculated by using GraphPad Prism software 9.3.
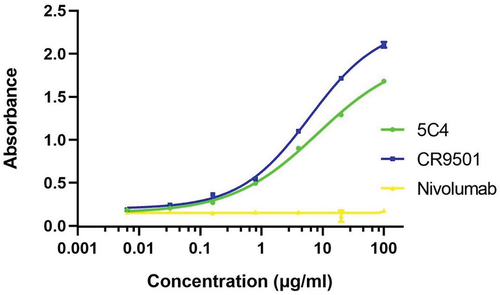
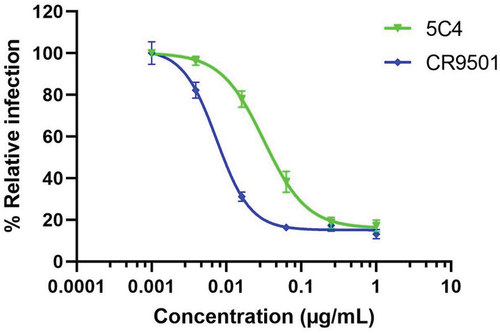
Figure 6. The neutralizing activity of plant-produced anti-RSV mAbs. Both plant-produced mAbs show a dose-dependent viral inhibitory effect. Error bars denote standard deviation of triplicate samples. The inhibitory concentration (IC50) was calculated using a normalized response with a variable slope in GraphPad Prism software version 9.3.
Mab_HuVac_Supplemantary_data_Cleaned 17022024.docx
Download MS Word (149.3 KB)Data availability statement
All the data are available from the corresponding author upon request.
