Figures & data
Figure 1. Participant flow.
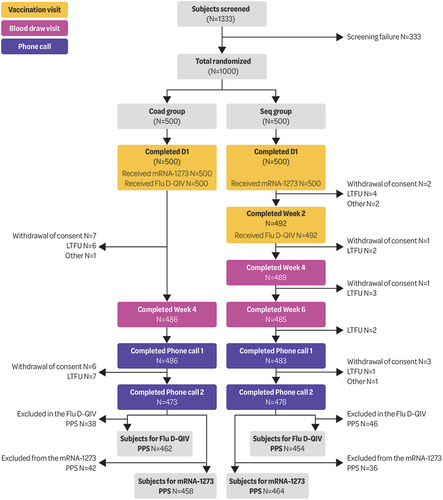
Table 1. Demographic characteristics of study participants (exposed set).
Figure 2. Adjusted geometric mean ratios (95% confidence intervals) of anti-spike antibodies and hemagglutinin inhibition 1 month post-vaccination (seq divided by Coad group) (per protocol set).
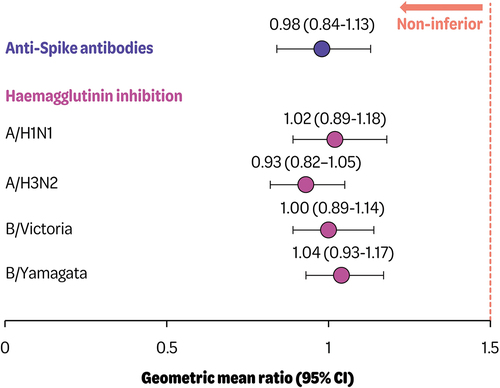
Figure 3. Percentage of solicited local and systemic adverse events reported per participant after the mRNA-1273 and QIV vaccinations (exposed set).
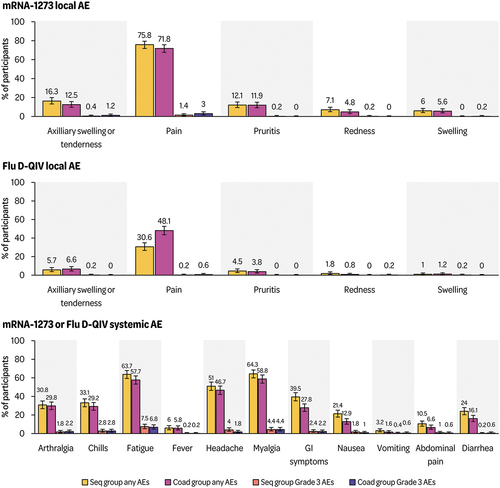
Table 2. Summary of published studies of mRNA COVID-19 booster doses co-administered with influenza vaccines.
Supplemental online material_revised_clean.docx
Download MS Word (49.6 KB)Data availability statement
Please refer to GSK weblink to access GSK’s data sharing policies and as applicable seek anonymized subject level data via the link https://www.gsk-studyregister.com/en/.
