Figures & data
Figure 1. Trial profile. The simultaneous group, receive QIIV at the same time as the first dose of COVID-19 vaccine; the non-simultaneous group, receive QIIV at 14 days after the second dose of COVID-19 vaccine; QIIV, quadrivalent inactivated influenza vaccine; FAS (the full-analysis set) was defined as subjects who completed QIIV vaccination after randomization into groups, completed pre-vaccination blood collection, and had valid pre-vaccination immunogenicity results; PPS (the per-protocol set) was defined as subjects who completed protocol-specified QIIV vaccination and COVID-19 vaccination, completed pre-vaccination and 30-day post-QIIV vaccination blood collection, and had valid immunogenicity results; SS (the safety set) was defined as subjects who had received at least one dose of vaccine; “*FAS,” one subject assigned to the non-simultaneous group was actually inoculated according to the simultaneous group procedure, and this subject was categorized as the non-simultaneous group in the FAS analysis and the simultaneous group in the PPS and SS analyses.
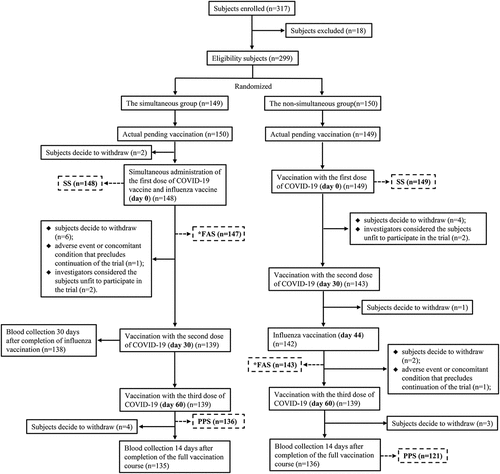
Table 1. Baseline characteristics of participants in the full-analysis set (FAS).
Table 2. Titer of QIIV antibodies for participants in the per-protocol set (PPS) before vaccination.
Table 3. The QIIV antibody titer for participants in the PPS dataset 30 days after QIIV vaccination.
Table 4. The seroconversion rates of antibodies after 30 days of QIIV vaccination (PPS).
Table 5. The seroprotection rate of the antibodies 30 days after QIIV vaccination (PPS).
Figure 2. The distribution of antibody titers (H1N1, H3N2, Yamagata, and Victoria) before and 30 days after QIIV vaccination (PPS). (a) H1N1; (b) H3N2; (c) Yamagata; (d) Victoria. PPS, the per-protocol set; QIIV, quadrivalent inactivated influenza vaccine.
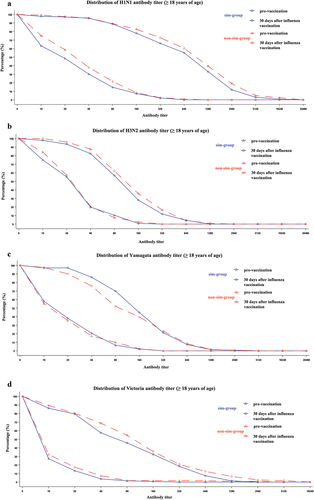
Table 6. The incidence of vaccine-related adverse events during vaccination.
