Figures & data
Table 1. Northern Hemisphere vaccine composition and reports of antigenic drift or egg-adapted mutations in the literature analysis of Northern Hemisphere 2011–2020 influenza seasons.
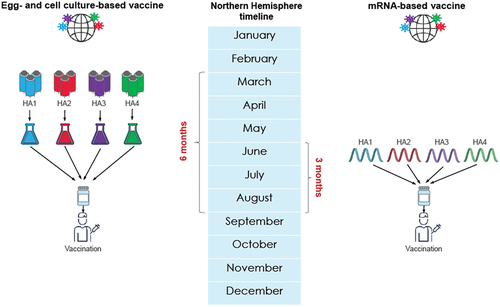
Figure 1. Influenza vaccine effectiveness, vaccine match, and strain prevalence by Northern Hemisphere influenza season and geographic region.
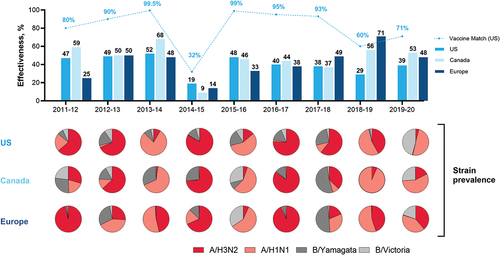
Figure 2. Influenza vaccine manufacturing using egg-based, cell culture-based, and mRNA-based platforms.