Figures & data
Figure 1. Characterization of membrane vesicles of different clb mutant strains.
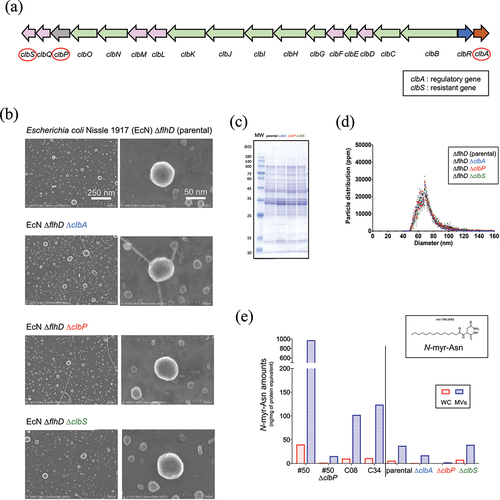
Figure 2. Mucosal adjuvanticity of MVs of different clb mutant strains.
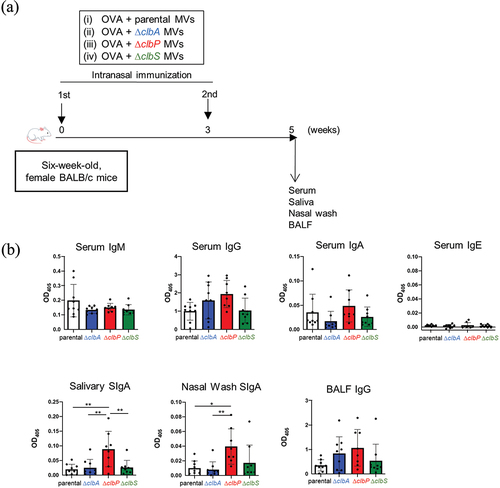
Figure 3. Characterization of MVs of parental and clbP mutant strains expressing pneumococcal serotype 14 capsular polysaccharide.
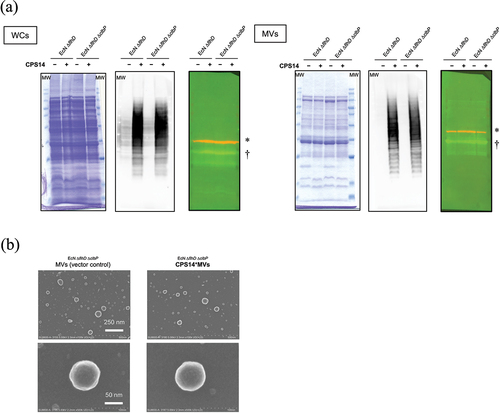
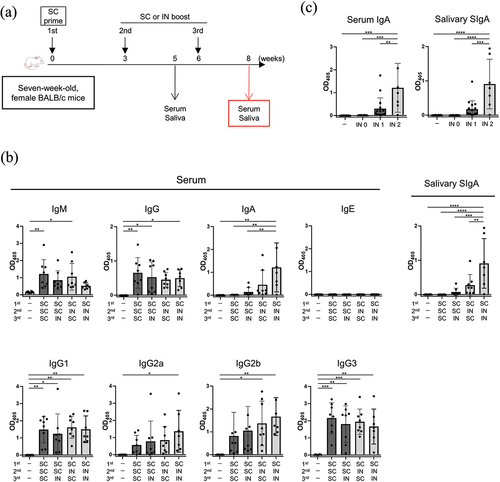
Figure 4. Humoral immune responses with two-dose vaccination regimen (Prime-Boost).
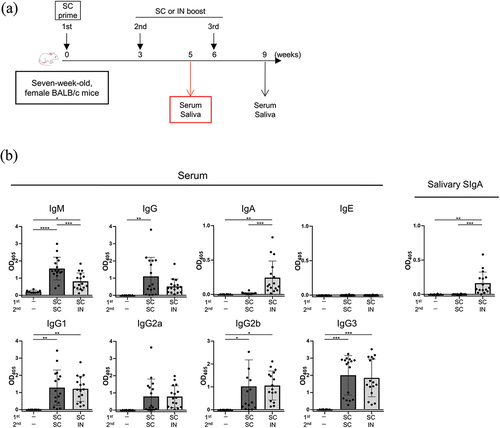
Figure 5. Humoral immune responses with three-dose vaccination regimen (prime-boost-additional boost).