Figures & data
Figure 1. Cumulative global cases and deaths of human infections with H5N1, H7N9, and H9N2 avian influenza viruses reported to the World Health Organization (WHO). Cases and deaths are cumulative from the first reported case of each virus subtype in humans until February 22, 2024. Numbers on top of the bars indicate how many cases and deaths have been reported to the WHO. Note: as of February 22, 2024, two deaths have been reported from H9N2 infections according to the WHO. Due to the limited scale of the figure, these deaths are not visually represented, but the number is included in the graph.
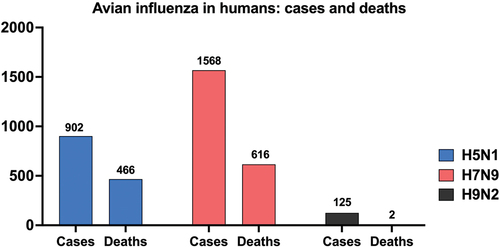
Figure 2. (a) H5N1, H7N9, and H9N2 influenza subtypes from avian sources can infect humans and cause disease. Sustained human-to-human transmission of these subtypes has not been observed but is required to the emergence of pandemic viruses which could further spread within the human population. (b) vaccine development and vaccination of humans can prevent infections and the generation of pandemic viruses, improving pandemic preparedness and management against avian influenza viruses. This figure was created with BioRender.com.
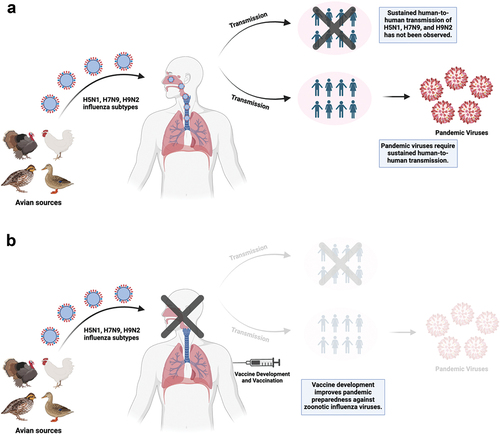
Table 1. Available candidate vaccine viruses for pandemic preparedness against the H5N1, H7N9, and H9N2 subtypes for the northern hemisphere influenza seasons 2024–2025.
Figure 3. Summary of animal data for H5N1, H7N9, and H9N2 vaccines tested in mice, ferrets, and non-human primates. The figure categorizes vaccines by platform (inactivated, live attenuated, and alternative) and uses color-coded boxes for each category. It is important to note that only data from vaccines discussed in this review for each animal model are included. While other platforms exist, they are not discussed in detail within this manuscript. This figure was created with BioRender.com.
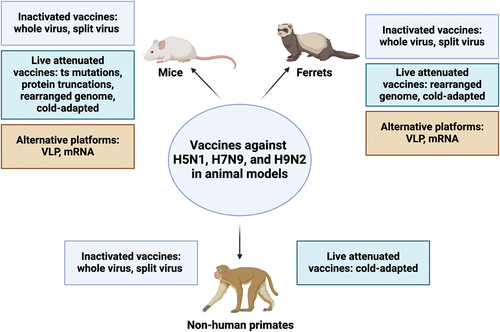
Table 2. Summary of clinical trials for H5N1, H7N9, and H9N2 avian influenza vaccines.
