Figures & data
©2024 Taylor & Francis Group, LLC
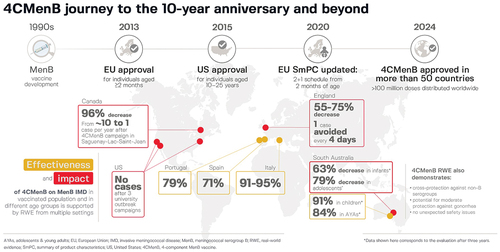
Figure 1. Road map leading to the regulatory approval and distribution of 4CMenB in more than 50 countries worldwideCitation9–11 and to the inclusion of 4CMenB in national and regional immunization programs since initial registration.
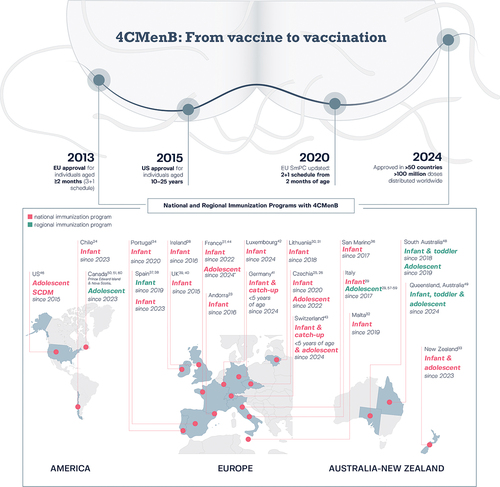
Figure 2. Recommendations in 16 national immunization programs for the coadministration (co-adm) of routine childhood vaccines with 4CMenB and the use of prophylactic paracetamol (known as acetaminophen in the United States).Citation52,Citation53
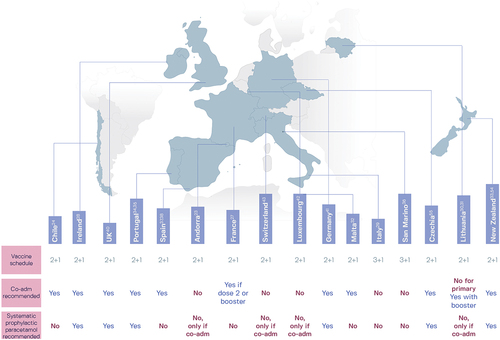
Figure 3. Real-world evidence of the effectiveness, impact, and safety of 4CMenB.

Figure 4. Observed and projected serogroup B invasive disease cases in South Australia before (2003–2016) and after 4CMenB vaccination of students aged 16–19 years as part of the ‘B part of It’ program.Citation78
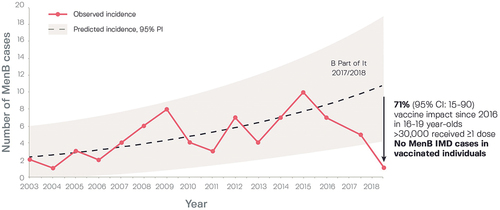
Figure 5. Ongoing and completed studies on the potential for 4CMenB to protect against Neisseria gonorrhoeae infection.
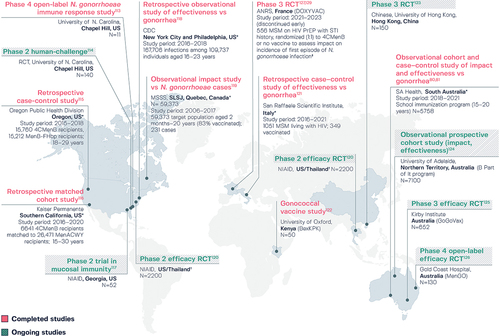
Figure 6. Potential coverage of 4CMenB against MenB strains circulating worldwide, as predicted by Meningococcal Antigen Typing System (MATS).
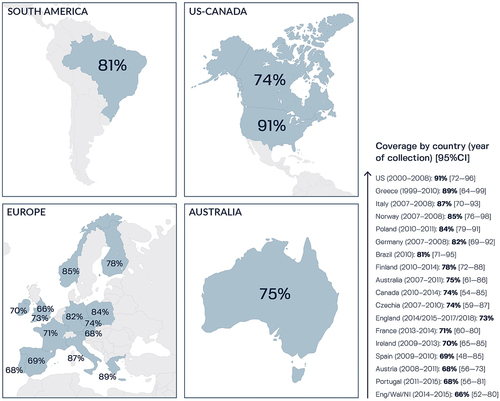
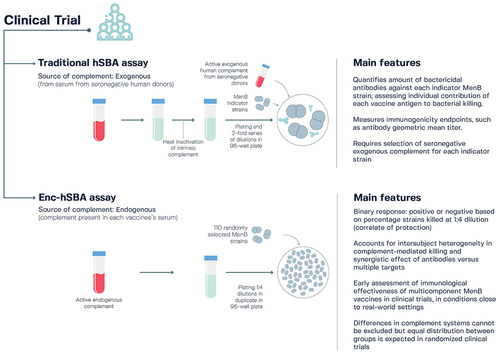
Figure 7. Characteristics of the traditional human serum bactericidal antibody (hSBA) assay and the endogenous complement hSBA (enc-hSBA) in relation to the assessment of meningococcal serogroup B (MenB) vaccines.Citation159,Citation184–186.