Figures & data
Figure 1. Chronology of all the development and activities carried out in the 2023-2024 RSV immunization campaign until December 31, 2023.
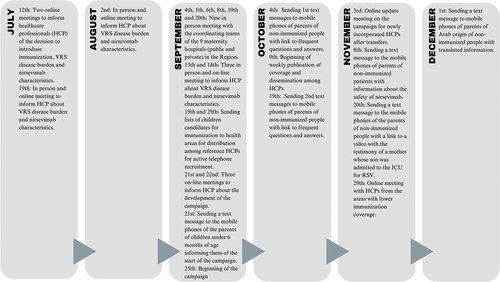
Table 1. Sociodemographic data of immunized and non-immunized children, born before the beginning of the campaign or during it.
Figure 2. Frequency distribution of immunization. (a) Days of life at immunization of all the children born from April 1, 2023 to March 31, 2024. (b) Days of life at immunization of children born during the campaign, from September 25, 2023 to March 31, 2024. (c) Days of life at immunization of children born before the beginning of the campaign, from April 1 to September 24, 2023. (d) Days since the official beginning of the campaign that it took children born before the beginning of the campaign, from April 1 to September 24, 2023, to be immunized.
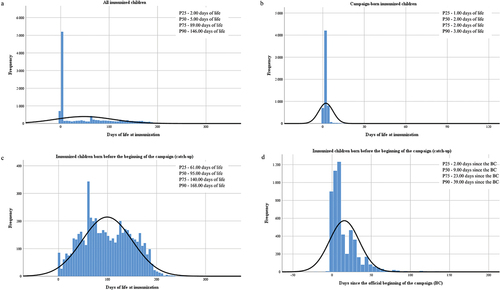
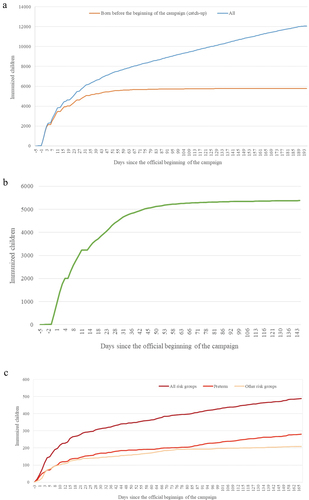
Figure 3. Cumulative immunization rate with nirsevimab. (a) Comparison between the immunization rate of all children (blue) compared to those born before the start of the season (orange), corresponding to the catch-up. (b) Evolution of the cumulative immunization rate of healthy children born before the start of the campaign. (c) Evolution of the cumulative immunization rate of children with risk conditions born before the start of the campaign, both globally (maroon) and comparatively between the group of premature babies less than 12 months of age (light red) and the rest of the risk conditions under 24 months of age (peach).