Figures & data
Figure 1. The antibody titer in camel serum and the schematic diagram of the VHH-hFc. (a) The specific antibody titer against TeNT-Hc in camel serum was detected before immunization, after the fourth immunization and the fifth immunization. (b) The schematic diagram of the VHH-hFc.
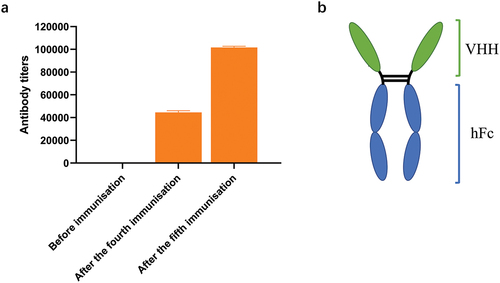
Table 1. TeNT-Hc phage nanobody library panning enrichment analysis.
Figure 2. Evaluation of the basic properties of the chimeric heavy chain antibodies. (a) Fourteen chimeric heavy chain antibodies analyzed by SDS-PAGE under non-reducing conditions (b) Fourteen chimeric heavy chain antibodies analyzed by SDS-PAGE under reducing conditions. (c) ELISA binding assay of the chimeric heavy chain antibodies to TeNT-Hc proteins. (d) The binding characteristics of the chimeric heavy chain antibodies. The 96-well plates were coated with different antigens (200 ng per well) and incubated with the candidate antibodies. 3% skimmed milk was used as the control. AHc, BHc, EHc, and FHc were the Hc domain of botulinum neurotoxins. TL-HN was another fragment of the full-length tetanus toxin, and 7Fibre was the fiber protein of human adenovirus 7.
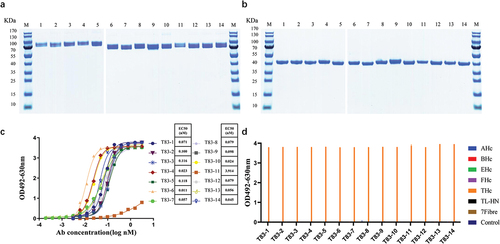
Table 2. The affinity between antibodies and TeNT-Hc determined by bio-layer interferometry.
Figure 3. The toxin neutralization capacity of the chimeric heavy chain antibodies. (a) A fixed dose of 10 μg of antibodies and 20 × LD50 TeNT was mixed, followed by intraperitoneal injection into mice, with 4 mice in each group. The time of death and the number of surviving mice were observed once a day, and the percentage of surviving mice was plotted for each antibody. A1 was a neutralizing antibody against BoNT/A and served as an unrelated control. (b) Four neutralizing antibodies at doses of 10 µg, 5 µg, 2.5 µg, 1.25 µg, 0.625 µg, and 0.3125 µg were mixed with 20 × LD50 TeNT and then injected intraperitoneally into mice, with four mice in each group. The time of death and the number of surviving mice were observed once a day, and the percentage of surviving mice was plotted for each antibody. All mice in the negative control group died within 48 h after injection.
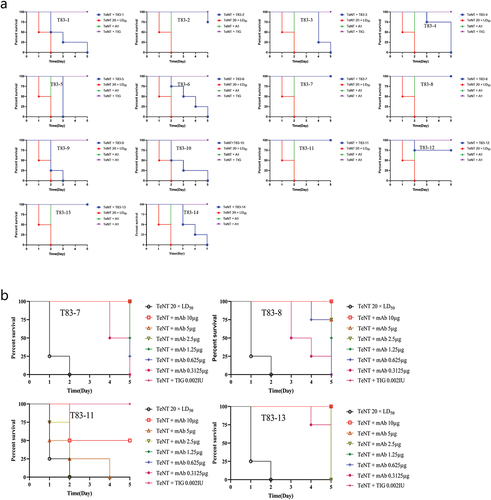
Table 3. Prophylactic efficacy of antibodies against TeNT in a mouse model.
Table 4. Therapeutic efficacy of antibodies against TeNT in a mouse model.
Figure S1.tif
Download TIFF Image (28.7 MB)Supplementary Material.docx
Download MS Word (804.5 KB)Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
