Figures & data
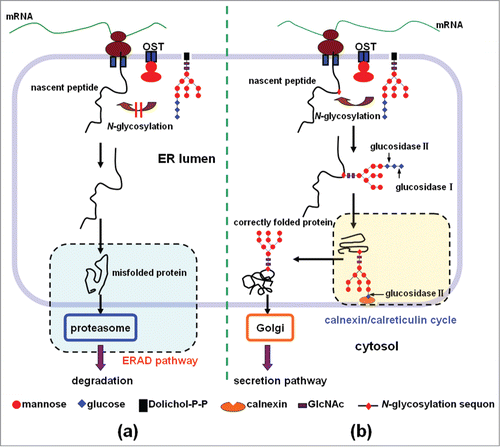
Figure 1. The role of N-glycosylation in the process of protein folding in the ER and secretion. (a) Lack of the N-glycosylation of the nascent peptide often leads to the protein misfolding. The misfolded protein will be subjected to ER-associated degradation (ERAD), and finally degraded in the cytoplasm by the proteasome. (b) N-glycosylation of the nascent peptide at the proper sites facilitates the protein folding and secretion. In the introduced sequon of Asn-Xaa-Thr/Ser (Xaa is any amino acid except proline, Thr is threonine, and Ser is serine), a lipid-linked oligosaccharide unit (Glc3Man9GlcNAc2) is transferred co-translationally to an Asn residue, and the oligosaccharide unit is then trimmed to Glc1Man9GlcNAc2 by glucosidase I and glucosidase II. The resulting glycoprotein enters the calnexin/calreticulin cycle to become properly folded, and exit the ER, and then go to the Golgi apparatus for secretion.