Figures & data
Figure 1. Distribution of 104, 303 XDH gene sequences retrieved from GenBank database as of April 9th, 2016. There are 96,077 bacterial XDHs (93.25%), 7513 eukaryotic XDHs (7.2%), 643 archaea XDHs (0.62%), 61 metagenomic XDHs (0.058%), 6 virus XDHs and 3 synthetic XDHs. In the bacteria domain, the dominant class is Proteobacteria, and the biggest subclass is the gamma Proteobacteria. This subclass consists mainly of the Pseudomonadales (18347) and Enterobacteria (18329) clusters, which are dominated by Pseudomonas genus (15570) and Escherichia genus (13258), respectively. In the eukaryotes domain, animals are the predominant contributor, which takes up 57.81% (4343). In the archaea domain, Sulfolobales genus with 335 species takes up the biggest sector (52.1%), of which Sulfolobus (278) is the majority genus. The number of the records in each class and its percentage that was rounded up are shown in each sector of the composite bar chart.
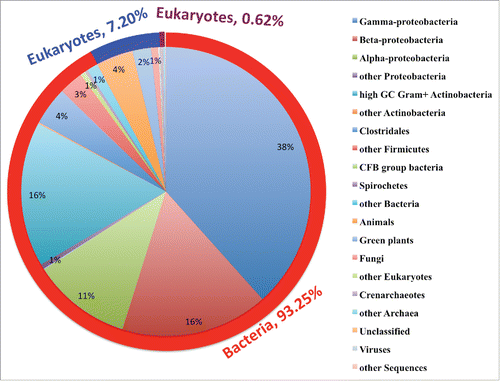
Table 1. Amino acid sequence similarity (identity) of [2Fe-2S], FAD and Moco domains of 9 representative XDHs.
Figure 2. Graphical representation (sequence logo) of amino acid sequence conservation of Xanthine dehydrogenase [2Fe-2S] (A), FAD (B) and Moco domains (C) derived from a sequence alignment of 9 representative species. The species are 2 eukaryotes, Bos taurus and Arabidopsis thaliana, 3 eubacteria, which are Rhodobacter capsulatus B10, Acinetobacter baumannii CICC10254 and Escherichia coli K-12, one archaebacteria, Pyrobactulum neutrophilum V24Sta, and 3 metagenomes, which are uncultured Archaea, Leaf litter and marine bacteria. The multiple sequence alignment of the [2Fe-2S] domains correspond to the 1–166 fragment of B. taurus XDH (GI: 27806775), 1–237 fragment of A. thaliana XDH1 (GI: 332661034), 1–152 fragment of R. capsulatus B10XDHA (GI: 2956674), 1–176 fragment ofA. baumannii CICC10254 (GI: 966039133), 1–159 fragment of E. coli K-12 XDHC (GI: 16130770), 1–148 fragment of P. neutrophilum V24Sta (2Fe-2S) binding domain protein (GI: 170934475), 1–228 fragment of Leaf litter metagenomic XDH [2Fe-2S] binding domain (GI: 534502715), 1–169 fragment of Uncultured marine microorganism HF4000 XDH putative FAD binding domain (GI: 167041895), and 1–157 fragment of Uncultured Archaea metagenomic XDH [2Fe-2S] binding domain (GI: 452077216). The FAD domains correspond to the 228–541 fragment of B. taurus XDH (GI: 27806775), 256–566 fragment of A. thaliana XDH1 (GI: 332661034), 174–463 fragment of R. capsulatus B10XDHA (GI: 2956674), 198–499 fragment of A. baumannii CICC10254 XDHA (GI: 966039133), E. coli K-12 XDHB with a length of 292 AA (GI: 16130769), P. neutrophilum V24Sta FAD domain protein with a length of 272 AA (GI: 170934475), Leaf litter metagenomic XDH FAD domain with a length of 323 AA (GI: 534502714), 192–479 fragment of uncultured marine microorganism HF4000 XDH putative FAD binding domain (GI: 167041895), and 1–282 fragment of Uncultured Archaea metagenomic XDH FAD domain (GI: 452077215). The Moco domains are the 556–1332 fragment of B. taurus XDH (GI: 27806775), 581–1361 fragment of A. thaliana XDH1 (GI: 332661034), R. capsulatus B10XDHB with a length of 777 AA (GI: 13397863), A. baumannii CICC10254 XDHB with a length of 792 AA (GI: 966039134), E. coli K-12 XDHA with a length of 752 AA (GI: 16130768), P. neutrophilum V24Sta FAD domain protein with a length of 724 AA (GI: 170934473), Leaf litter metagenomic XDH FAD domain with a length of 734 AA (GI: 534502713), uncultured marine microorganism HF4000 XDH putative molybdopterin binding domain with a length of 764 AA (GI: 167041894), and uncultured Archaea metagenomic XDH molybdopterin binding subunit with a length of 780 AA (GI: 452077214). Each logo consists of stacks of symbols, one stack for each position in the sequence. The overall height of the stack indicates the sequence conservation at that position, while the height of symbols within the stack indicates the relative frequency of each amino or nucleic acid at that position. Amino acids are colored according to their chemical properties: polar amino acids (G,S,T,Y,Q,N) are black except the C which is red, basic (K,R,H) green, acidic (D,E) blue and hydrophobic (A,V,L,I,P,W,F,M) amino acids are purple. The most conserved amino acids in the [2Fe-2S], FAD and Moco domains are indicated by hexagram, triangles and diamonds, and pentagram, respectively. The eight identical cysteines consist of the 2 [2Fe-2S] cluster binding motifs of [2Fe-2S] domain: C-X4-C-X2-C-Xn-C and C-X2-C-Xn-C-X1-C, where X indicates any amino acid, and n means any number of amino acids. The triangles indicate the 4 identical amino acids residues in the 4 conserved FAD binding motifs, and the diamonds show the conservative residue sites that affect the FAD electrostatic environment. The red box shows the loop423–433 residues (numbering in bovine XDH) that dominate the electrostatic potential of FAD binding, resulting in different electron transfer efficiency from Fe/S cluster to NAD. The pentagram stars indicate the conserved active site catalytic residues. Using the bovine XDH numbering, Glu802 binds the substrate and stabilizes the transition state, Glu1261 is the catalytic base, Arg880 and Thr1010 bind the substrate and decrease the reaction activation energy, Phe914 and Phe1009 orientate the substrate via π–π stacking, Val1011 is the key residue channeling the substrate, and Gln758 is responsible for releasing the product.
![Figure 2. Graphical representation (sequence logo) of amino acid sequence conservation of Xanthine dehydrogenase [2Fe-2S] (A), FAD (B) and Moco domains (C) derived from a sequence alignment of 9 representative species. The species are 2 eukaryotes, Bos taurus and Arabidopsis thaliana, 3 eubacteria, which are Rhodobacter capsulatus B10, Acinetobacter baumannii CICC10254 and Escherichia coli K-12, one archaebacteria, Pyrobactulum neutrophilum V24Sta, and 3 metagenomes, which are uncultured Archaea, Leaf litter and marine bacteria. The multiple sequence alignment of the [2Fe-2S] domains correspond to the 1–166 fragment of B. taurus XDH (GI: 27806775), 1–237 fragment of A. thaliana XDH1 (GI: 332661034), 1–152 fragment of R. capsulatus B10XDHA (GI: 2956674), 1–176 fragment ofA. baumannii CICC10254 (GI: 966039133), 1–159 fragment of E. coli K-12 XDHC (GI: 16130770), 1–148 fragment of P. neutrophilum V24Sta (2Fe-2S) binding domain protein (GI: 170934475), 1–228 fragment of Leaf litter metagenomic XDH [2Fe-2S] binding domain (GI: 534502715), 1–169 fragment of Uncultured marine microorganism HF4000 XDH putative FAD binding domain (GI: 167041895), and 1–157 fragment of Uncultured Archaea metagenomic XDH [2Fe-2S] binding domain (GI: 452077216). The FAD domains correspond to the 228–541 fragment of B. taurus XDH (GI: 27806775), 256–566 fragment of A. thaliana XDH1 (GI: 332661034), 174–463 fragment of R. capsulatus B10XDHA (GI: 2956674), 198–499 fragment of A. baumannii CICC10254 XDHA (GI: 966039133), E. coli K-12 XDHB with a length of 292 AA (GI: 16130769), P. neutrophilum V24Sta FAD domain protein with a length of 272 AA (GI: 170934475), Leaf litter metagenomic XDH FAD domain with a length of 323 AA (GI: 534502714), 192–479 fragment of uncultured marine microorganism HF4000 XDH putative FAD binding domain (GI: 167041895), and 1–282 fragment of Uncultured Archaea metagenomic XDH FAD domain (GI: 452077215). The Moco domains are the 556–1332 fragment of B. taurus XDH (GI: 27806775), 581–1361 fragment of A. thaliana XDH1 (GI: 332661034), R. capsulatus B10XDHB with a length of 777 AA (GI: 13397863), A. baumannii CICC10254 XDHB with a length of 792 AA (GI: 966039134), E. coli K-12 XDHA with a length of 752 AA (GI: 16130768), P. neutrophilum V24Sta FAD domain protein with a length of 724 AA (GI: 170934473), Leaf litter metagenomic XDH FAD domain with a length of 734 AA (GI: 534502713), uncultured marine microorganism HF4000 XDH putative molybdopterin binding domain with a length of 764 AA (GI: 167041894), and uncultured Archaea metagenomic XDH molybdopterin binding subunit with a length of 780 AA (GI: 452077214). Each logo consists of stacks of symbols, one stack for each position in the sequence. The overall height of the stack indicates the sequence conservation at that position, while the height of symbols within the stack indicates the relative frequency of each amino or nucleic acid at that position. Amino acids are colored according to their chemical properties: polar amino acids (G,S,T,Y,Q,N) are black except the C which is red, basic (K,R,H) green, acidic (D,E) blue and hydrophobic (A,V,L,I,P,W,F,M) amino acids are purple. The most conserved amino acids in the [2Fe-2S], FAD and Moco domains are indicated by hexagram, triangles and diamonds, and pentagram, respectively. The eight identical cysteines consist of the 2 [2Fe-2S] cluster binding motifs of [2Fe-2S] domain: C-X4-C-X2-C-Xn-C and C-X2-C-Xn-C-X1-C, where X indicates any amino acid, and n means any number of amino acids. The triangles indicate the 4 identical amino acids residues in the 4 conserved FAD binding motifs, and the diamonds show the conservative residue sites that affect the FAD electrostatic environment. The red box shows the loop423–433 residues (numbering in bovine XDH) that dominate the electrostatic potential of FAD binding, resulting in different electron transfer efficiency from Fe/S cluster to NAD. The pentagram stars indicate the conserved active site catalytic residues. Using the bovine XDH numbering, Glu802 binds the substrate and stabilizes the transition state, Glu1261 is the catalytic base, Arg880 and Thr1010 bind the substrate and decrease the reaction activation energy, Phe914 and Phe1009 orientate the substrate via π–π stacking, Val1011 is the key residue channeling the substrate, and Gln758 is responsible for releasing the product.](/cms/asset/00f9daa6-cacb-4ea0-a147-922fb8683b29/kbie_a_1206168_f0002_oc.gif)
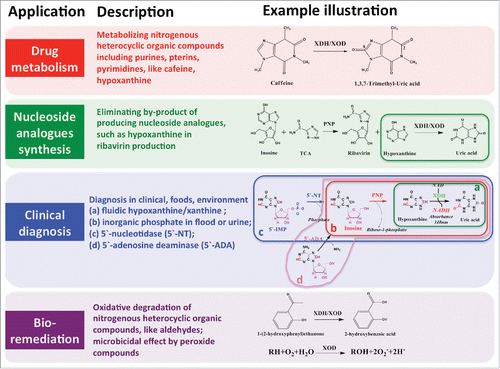
Figure 3. Biosynthesis of XDH and its conversion to XOD. (I) Biosynthesis of sulfurated molybdenum cofactor. Mo (molybdenum) is inserted in the MPT (molybdopterin or metal-containing pterin) to form the molybdenumcofactor (Moco) by molybdenum insertase, like Cnx1 in plants, Geph in Humans, or MogA and MoeA in E. coli. Then, Moco is sulfurated by the Moco sulfurase, that is ABA3 in plants or NifS in E. coli. The chaperone XdhC binds stoichiometric amount of Moco, interacts with NifS4 for the sulfuration of Moco, protects sulfurated Moco from oxidation, and further transfers to XDH. (II) Biosynthesis of active XDH. It involves 5 processes: 1) synthesis of apoproteins consisting of the 3 domains, depicted as S, M and L; 2) formation of heteromultimer apoprotein in the case of multi-subunit heteromultimer XDH; 3) insertion of 2 [2Fe-2S] and a FAD cofactor into the S and M domains respectively; 4) dimerization of apoprotein assembled with [2Fe-2S] and a FAD cofactors via the L domain; 5) insertion of sulfurated Moco into the L subunits with the help of chaperone protein XdhC. The Moco insertion is the last step to form active XDH and occurs after the formation of dimerization of multi-domain apoprotein or heteromultimer assembled with [2Fe-2S] and FAD cofactors. (III) Conversion from active XDH to XOD. The active XDH is reversibly converted into XOD by forming 2 cysteine disulfide bonds that causes the conformational rearrangement around the FAD domain to prefer the oxygen as electron acceptor. The irreversible conversion is carried out by limited proteolysis via trypsin or chymotrypsin. The five arrows marked with numbers indicate the possible keys for efficient production of active XDH/XODs.
![Figure 3. Biosynthesis of XDH and its conversion to XOD. (I) Biosynthesis of sulfurated molybdenum cofactor. Mo (molybdenum) is inserted in the MPT (molybdopterin or metal-containing pterin) to form the molybdenumcofactor (Moco) by molybdenum insertase, like Cnx1 in plants, Geph in Humans, or MogA and MoeA in E. coli. Then, Moco is sulfurated by the Moco sulfurase, that is ABA3 in plants or NifS in E. coli. The chaperone XdhC binds stoichiometric amount of Moco, interacts with NifS4 for the sulfuration of Moco, protects sulfurated Moco from oxidation, and further transfers to XDH. (II) Biosynthesis of active XDH. It involves 5 processes: 1) synthesis of apoproteins consisting of the 3 domains, depicted as S, M and L; 2) formation of heteromultimer apoprotein in the case of multi-subunit heteromultimer XDH; 3) insertion of 2 [2Fe-2S] and a FAD cofactor into the S and M domains respectively; 4) dimerization of apoprotein assembled with [2Fe-2S] and a FAD cofactors via the L domain; 5) insertion of sulfurated Moco into the L subunits with the help of chaperone protein XdhC. The Moco insertion is the last step to form active XDH and occurs after the formation of dimerization of multi-domain apoprotein or heteromultimer assembled with [2Fe-2S] and FAD cofactors. (III) Conversion from active XDH to XOD. The active XDH is reversibly converted into XOD by forming 2 cysteine disulfide bonds that causes the conformational rearrangement around the FAD domain to prefer the oxygen as electron acceptor. The irreversible conversion is carried out by limited proteolysis via trypsin or chymotrypsin. The five arrows marked with numbers indicate the possible keys for efficient production of active XDH/XODs.](/cms/asset/b28f0e67-a4b0-4771-b1d7-1680a9247922/kbie_a_1206168_f0003_oc.gif)
Table 2. Comparison of some commercial XODs and 2 bacterial XDHs.