Figures & data
Figure 1. The model complex of GluTR and GSA-AT. The V-shaped dimer protein is GluTR while the other dimer is GSA-AT.
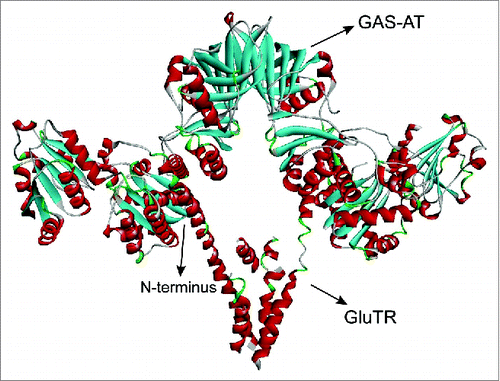
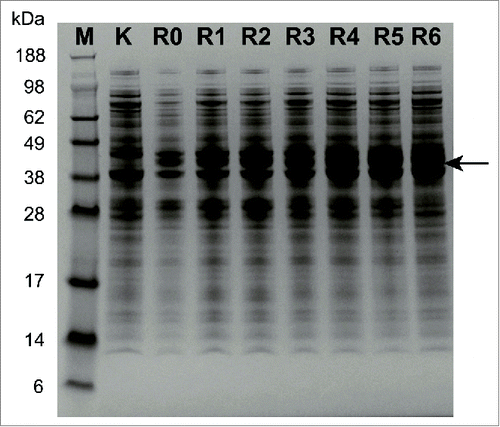
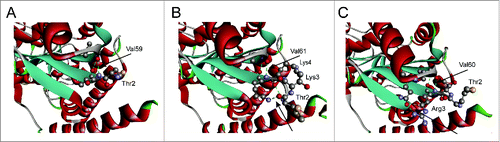
Figure 2. The illustration of GluTR variants with insertion of different numbers of lysine residues (A) and arginine residues (B). WT represented the wild-type GluTR.
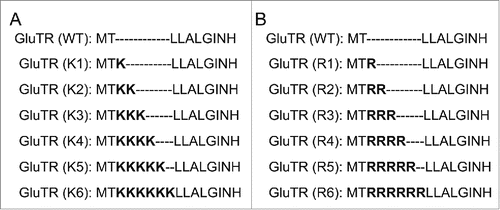
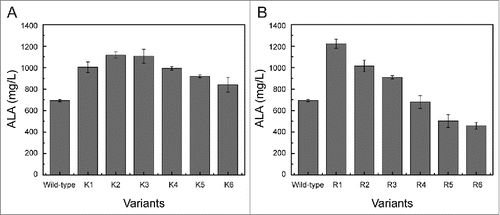
Figure 3. ALA production of the variants with inserting different lysine residues (A) and arginine residues (B).