Figures & data
Figure 1. Showing minimized energy structure. (A) Porcine α- amylase; (B) Acarbose; (C) Gallic acid; (D) Catechin; (E) Epicatechin; (F) Proanthocyanidin.
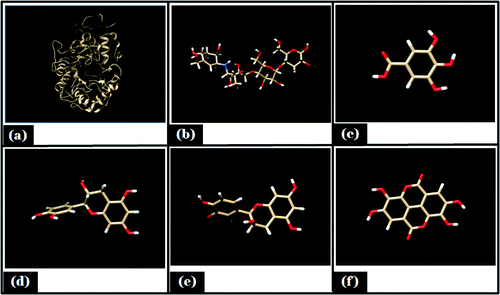
Table 1. Chemoprofiling of Vicia faba seed extracts in different solvents.
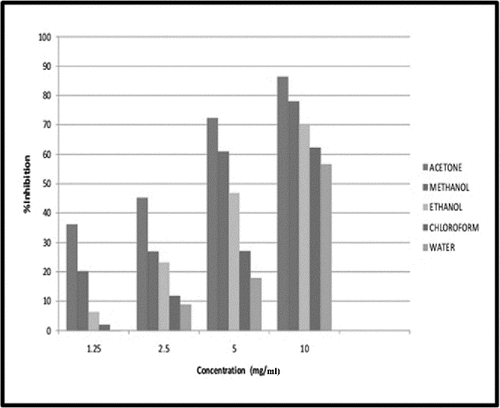
Table 2. IC50 values for α-amylase inhibitory potential of Vicia faba seed extracts.
Figure 3. Mode of inhibition of α-amylase by acetone and methanol seed extract (Michaelis- Menten plot).
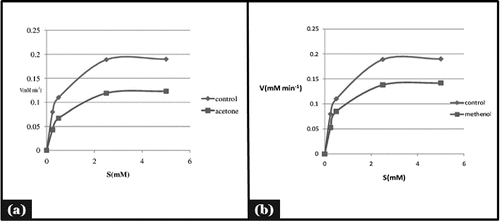
Figure 4. Mode of inhibition of α-amylase by acetone and methanol seed extract (Lineweaver-Burk plot).
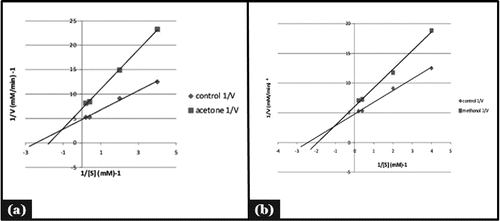
Table 3. Kinetic analysis of Vicia faba seed extract with respect to reference molecule.
Table 4. Total sugar.total phenol content of acetonic seed extract fraction.
Figure 7. Lig plot showing hydrogen bonding and hdrophobic interaction in different ligand, (A) Acarbose; (B) Epicatechein; (C) Gallic acid; (D) Proanthocyanidin; (E) Catechin with porcine α- amylase.
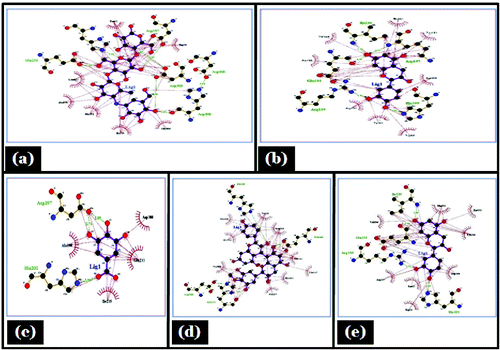
Figure 8. Showing autodock tool analysis of different types of interaction in different ligands. (A) Catechin; (B) Epicatechin; (C) Acarbose; (D) Proanthocyanidin; (E) Gallic acid with porcine α- amylase.
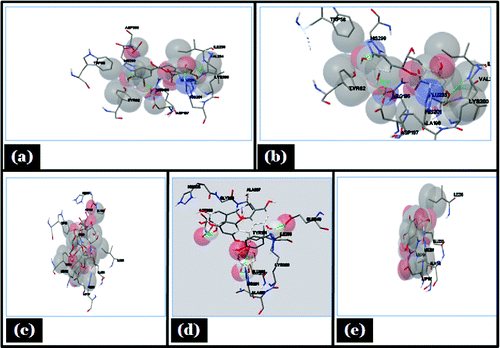
Table 5. Docking analysis of different ligands with porcine α amylase.