Figures & data
Table 1. Percent yield when different solvents were used (mg/100 g crude extract)
Table 2. Total phenolic, tannin, alkaloid and flavonoid contents in the petroleum ether Vaccinium macrocarpon extract
Table 3. Antioxidative profile of different variables in EtO-induced, toxicity-treated rats receiving an extract of Vaccinium macrocarpon.
Table 4. Active constituents of the plant extract
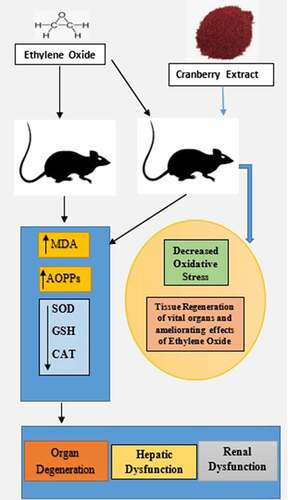
Figure 1. Ethylene oxide, an epoxide, has basically two sources, i.e., exogenous and endogenous. The burning of vegetation, the degradation of agricultural waste and refuse, and the incomplete combustion of fossil fuels are exogenous sources. Endogenous sources include the lipid peroxidation of unsaturated fats, the metabolism of ethylene to ethylene oxide by intestinal flora, the oxidation of heme in hemoglobin and free methionine. Whatever the source of production, the result of the encounter is absorption by the body, which can occur by three routes: inhalation, oral or dermal. After being taken up by any of these routes, ethylene oxide is widely distributed by the blood throughout the whole body. It is metabolized in tissues by two major pathways. One pathway is conjugation with GSH, resulting in the production of metabolites by a series of reactions, the end products of which are excreted in urine. During conjugation, the disruption of thiol (-SH) groups leads to the impaired function of GSH, that results in oxidative damage. The markers of oxidative stress that we measured were SOD, catalase, MDA and AOPP. The first two are enzymatic, while the latter two are non-enzymatic. The other pathway involves hydrolysis to form ethylene glycol, which later forms products such as oxalic acid, which induce nephrotoxicity, the chelation of Ca2+ in the bloodstream and the formation of Ca2+-oxalate crystals in the kidneys. In addition to ethylene glycol, 2-chloroethanol is formed, which causes DNA damage, tissue damage and organ degeneration
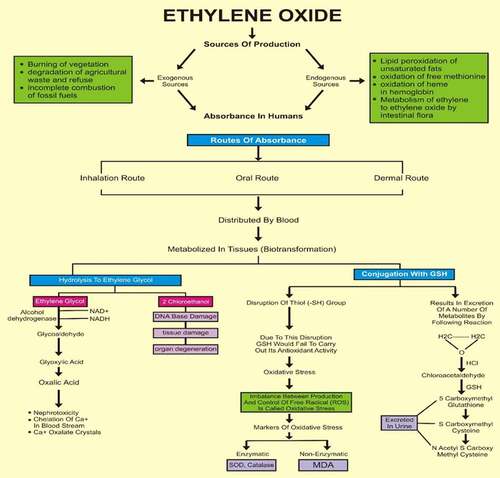
Figure 2. Histopathological examination of the hearts of rats before and after treating with cranberry (scale bar 35 microns with 40x magnification), indicating severe cardiac myocyte damage with a loss of myocardial fibers and vacuolated cells
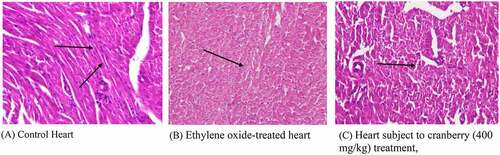
Figure 3. Histopathological examination of the kidneys of rats before and after treatment with cranberry (scale bar 35 microns with 40x magnification). Although the architecture of the kidney tissue remained normal, EtO dilation of renal tubules could be observed
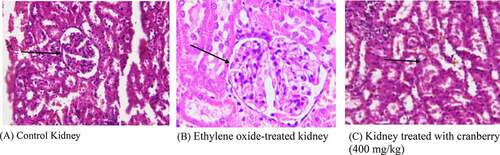
Figure 4. Histopathological examination of the livers of rats before and after treatment with cranberry (scale bar 35 microns with 40x magnification). Following exposure to EtO, the normal structural organization of the hepatic lobules indicated damaged cells with a loss of characteristic cord-like arrangements
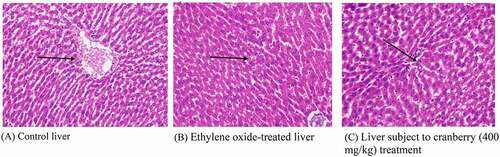
Figure 5. Histopathological examination of the lungs of rats before and after treatment with cranberry (scale bar 35 microns with 40x magnification). Changes can be observed in the architecture of connective tissues, smooth tissues and bronchioles in EtO-treated lung tissues
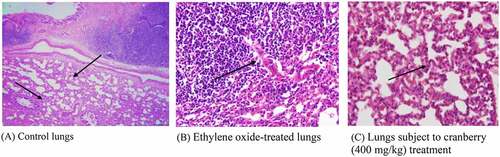
Figure 6. Histopathological examination of the stomach of rats before and after treating with cranberry (scale bar 35 microns with 40x magnification). The photomicrograph of the EtO-treated stomach clearly shows a decrease in the cytoplasmic content and a narrowing of the cells
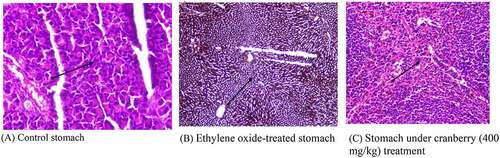
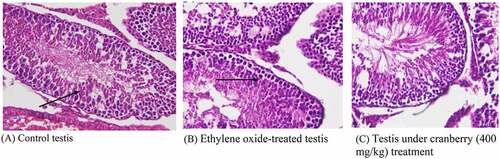
Figure 7. Histopathological examination of the testes of rats before and after treatment with cranberry (scale bar 35 microns with 40x magnification). Photomicrographs of EtO-treated testes indicated edema leading to the separation of the cell lining of the seminiferous tubules, the seizure of spermatogenesis, and focal necrosis