Figures & data
Figure 1. Screening of targets related to atherosclerosis and FMNT. (a) Venn diagram displays 39 overlapping genes between atherosclerosis- and FMNT-related target genes. (b) A PPI network of 39 intersection genes
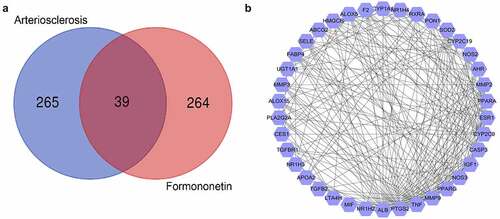
Figure 2. Biological function and pathway assays. (a) GO second class enrichment assay of 39 potential targets for FMNT against atherosclerosis. (b) Bubble chart showing the top 20 biological processes (BP) of GO terms. (c) Bubble chart of top 20 signaling pathways linked to FMNT against atherosclerosis
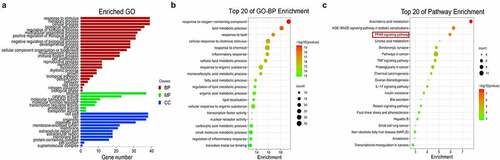
Figure 3. FMNT attenuates ox-LDL-induced viability inhibition in HUVECs. (a) HUVECs were treated with designated concentrations of FMNT for 24 h, followed cell viability determination. (b and c) HUVECs were treated with 40 μg/mL of ox-LDL for 24 h with or without 40 µM FMNT, followed by CCK-8 assay of cell viability (b) and EdU assay of cell proliferation (c). **P < 0.01, ***P < 0.001
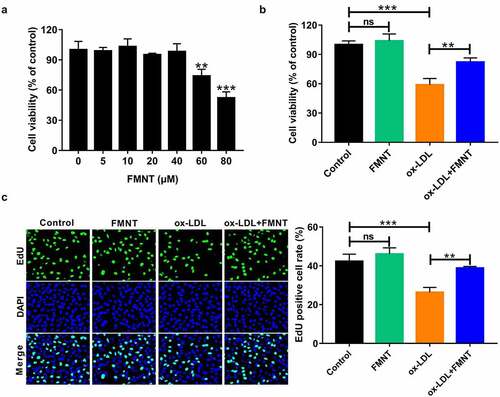
Figure 4. FMNT alleviates ox-LDL-induced inflammatory response in HUVECs. HUVECs were exposed to 40 μg/mL of ox-LDL for 24 h in the presence or absence of 40 µM FMNT. (a and b) The TNF-α and IL-1β levels in culture supernatant were determined by corresponding ELISA kits. (c) Western blot assays of COX-2 protein expression in HUVECs. ***P < 0.001
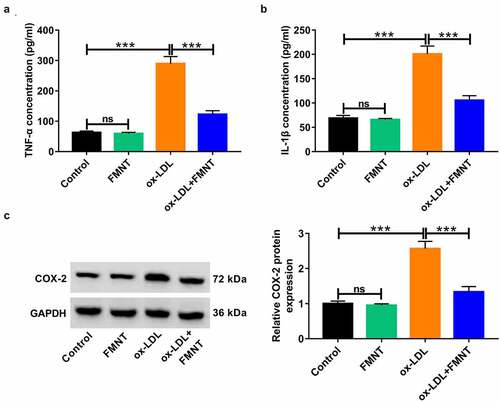
Figure 5. FMNT relieves oxidative stress and apoptosis in ox-LDL-treated HUVECs. HUVECs were stimulated with 40 μg/mL of ox-LDL for 24 h with or without 40 µM FMNT. (a and b) The MDA content and SOD activity in culture supernatant were measured with corresponding assay kits. (c) Intracellular ROS generation was examined by DCFH-DA staining. (d) Flow cytometry assays of apoptotic rate. (e) Western blot assays of eNOS and cleaved caspase-3 expression. *P < 0.05, **P < 0.01, ***P < 0.001
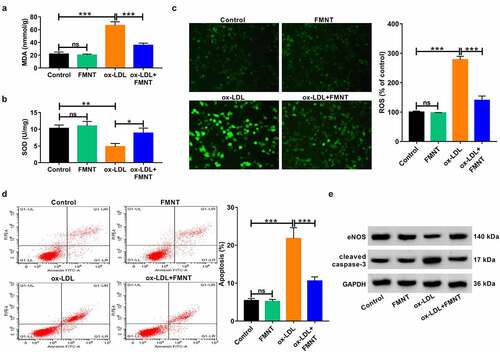
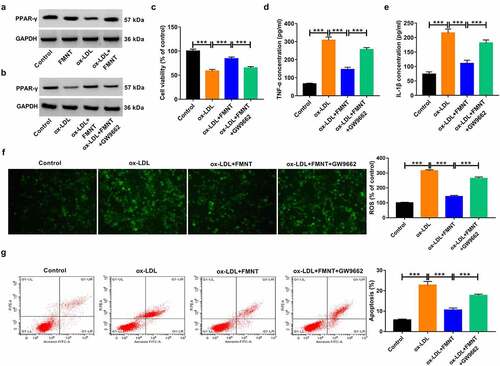
Figure 6. Inactivation of PPAR-γ signaling reversed the effects of FMNT on ox-LDL-induced endothelial injury. (a) Western blot assay was applied to evaluate the effect of FMNT on PPAR-γ expression in ox-LDL-treated HUVECs. (b-g) PPAR-γ antagonist (GW9662, 5 μM) was used to block PPAR-γ signaling in HUVECs prior to ox-LDL and FMNT treatment, followed by assays of PPAR-γ protein expression (b), cell viability (c), TNF-α and IL-1β level in culture supernatant (d and e), intracellular ROS level (f) and apoptotic rate (g). ***P < 0.001