Figures & data
Figure 1. LPS induces the apoptosis of NCM460 cells. (a) A CCK-8 assay was conducted to access the NCM460 cell viability after being treated with increasing levels of LPS (0, 5, 10, 15, 20 μg/ml). (b) The apoptosis of NCM460 cells was examined by flow cytometry analysis. (c) The expression of apoptosis-associated proteins (Bax, Bcl-2, C-caspase-3) in LPS-stimulated NCM460 cells was detected by western blot. ***p < 0.001
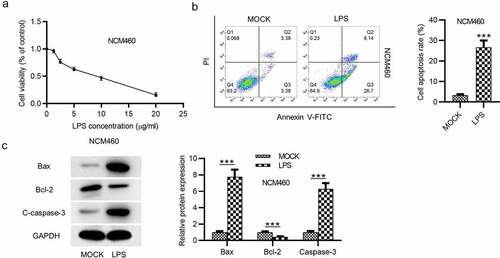
Figure 2. LPS induces epithelial barrier dysfunction of NCM460 cells. (a) Transepithelial electrical resistance (TEER) of NCM460 cells after LPS treatment was detected to analyze the barrier function. (b) Non-absorbable fluorescein isothiocyanate (FITC)-conjugated dextran was used to access the permeability of NCM460 cells. (c) The expression of tight junction proteins (ZO-1, occludin) in LPS-stimulated NCM460 cells was detected by western blot. (d and e) The RT-qPCR and ELISA were used to measure the mRNA expression of iNOS and the concentration of NO in LPS-treated NCM460 cells. ***p < 0.001
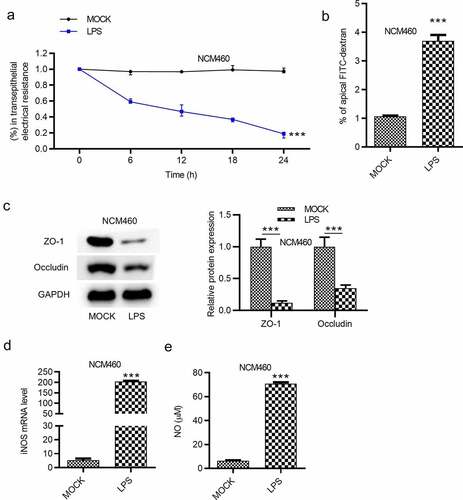
Figure 3. LPS induces the inflammatory response of NCM460 cells. (a and b) The concentrations of IL-6 and TNF-α in NCM460 cells after indicated treatment were measured using ELISA. ***p < 0.001
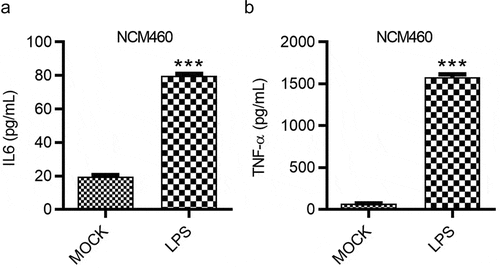
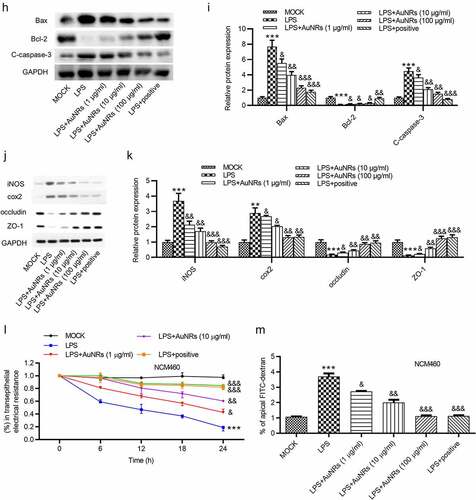
Figure 4. AuNPs ameliorate the LPS-induced NCM460 cell injury. (a–d) The concentrations of IL-6, TNF-α and NO and mRNA expression of iNOS in NCM460 cells treated with LPS or LPS in combination with elevated concentrations of AuNPs (1, 10, 100 μg/ml). (e) A CCK-8 assay was performed to examine the viability of NCM460 cells treated with LPS alone or in combination with different concentrations of AuNPs. (f and g) The apoptosis of NCM460 cells treated with LPS alone or in combination with different concentrations of AuNPs was examined by flow cytometry analysis. (h and i) The protein levels of Bax, Bcl-2, C-caspase-3 were analyzed by western blot. (j and k) The protein levels of tight junction proteins (occludin, ZO-1) and pro-inflammatory factors (iNOS, COX2) were detected by western blot. (l) The barrier function of NCM460 cells treated with LPS or LPS with AuNPs was examined by transepithelial electrical resistance (TEER) assay. (m) The permeability of NCM460 cells after treatment of LPS or LPS and AuNPs was assessed by paracellular solute FITC-dextran. *p < 0.05, ***p < 0.001, &p < 0.05, &&p < 0.01, &&&p < 0.001
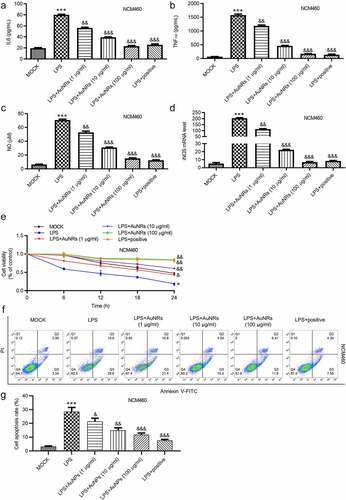
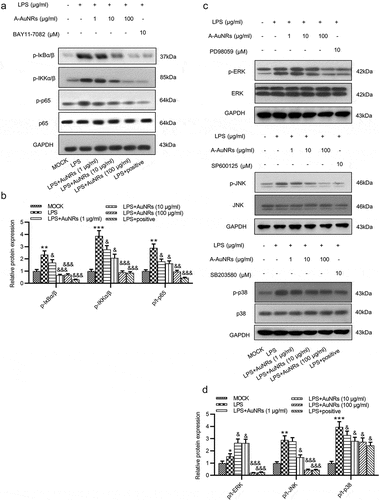
Figure 5. AuNPs inhibits the activation of the NF-κB and ERK/JNK signaling pathways. (a–d) Western blot was used to examine the levels of key proteins on the NF-κB and ERK/JNK signaling such as p-IκBα/β, p-IKKα/β, p-p65, p65, p-ERK, ERK, p-JNK, JNK, p-p38 and p38 after LPS treatment alone or in combination with AuNPs in NCM460 cells. BAY11-7082 was used as the inhibitor of NF-κB, while the PD98059 was used as inhibitor of ERK/JNK. *p < 0.05, **p < 0.01, ***p < 0.001, &p < 0.05, &&p < 0.01, &&&p < 0.001