Figures & data
Figure 1. MEGF9 is a target of miR-7. (a) The sequence of MEGF9 contains a nucleotide sequence complementary to miR-7. (b) Dual‐luciferase reporter assay and (c) Biotin-based RNA immunoprecipitation assay for binding interaction between miR-7 and MEGF9. ***P < 0.001
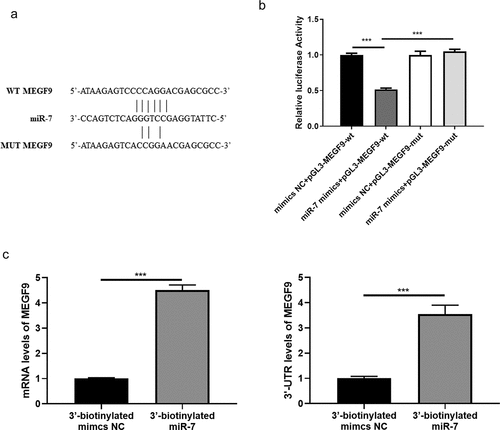
Figure 2. MEGF9 is higher expressed in OA tissues and interacts with EGFR
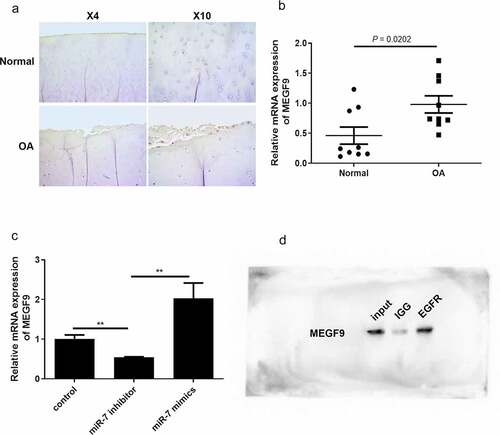
Figure 3. miR-7 regulates MEGF9 to affect cartilage degradation related proteins. The western blot and quantitative analysis of MEGF9, EGFR, MMP13 and ADAMTS-5 expression regulated by miR-7 in OA cells with different treatments. *P < 0.05, **P < 0.01, ***P < 0.001
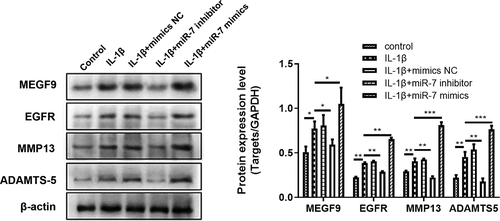
Figure 4. Overexpression of MEGF9 promotes cartilage degradation and activates PI3K/AKT signaling pathway. The mRNA levels of MEGF9 (a), EGFR (b), MMP13 (c), and ADAMTS-5 (d); and the western blot and quantitative analysis (e) of MEGF9, EGFR, MMP13, ADAMTS-5, p-PI3K and p-AKT in OA cells with different treatments. *P < 0.05, **P < 0.01, ***P < 0.001
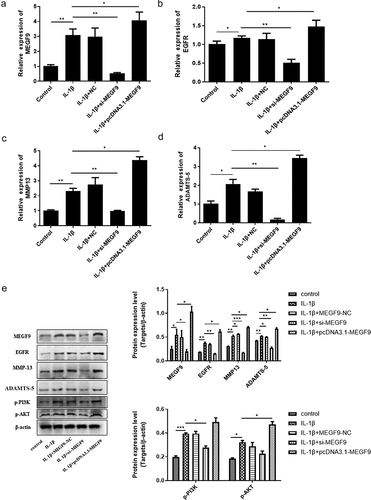
Figure 5. LY294002 rescues the effect of MEGF9 on cartilage degradation. The mRNA levels of MEGF9 (a), EGFR (b), MMP13 (c), and ADAMTS-5 (d); and the western blot and quantitative analysis (e) of MMP13 and ADAMTS-5 in OA cells with different treatments. *P < 0.05, **P < 0.01, ***P < 0.001
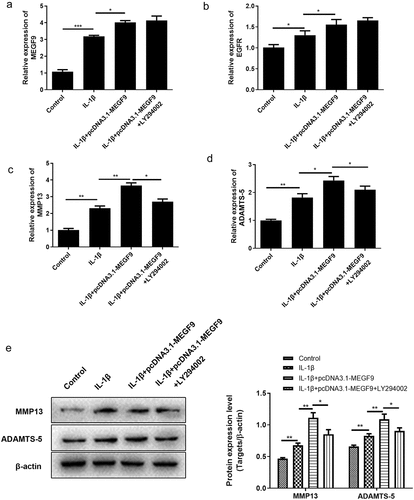
Figure 6. MEGF9 was highly expressed in OA mice with miR-7 mimics treatment. The immunohistochemical analysis of MEGF9 in OA mice
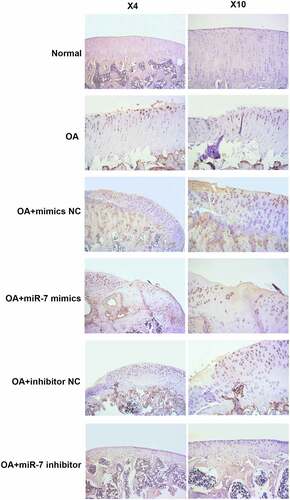
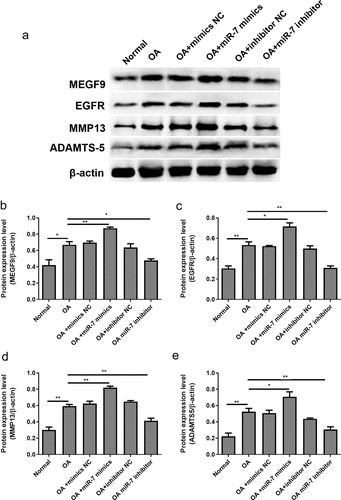
Figure 7. miR-7 mediated MEFG9 to regulate the expression of cartilage degradation-related proteins in OA mice. (a) The western blot results of MEGF9, EGFR, MMP13, and ADAMTS-5; and quantitative analysis of MEGF9 (b), EGFR (c), MMP13 (d) and ADAMTS-5 (e) in OA mice with different treatments. *P < 0.05, **P < 0.01, ***P < 0.001
Data availability statement
The data that support the findings of this research are available from the corresponding author upon reasonable request.
