Figures & data
Figure 1. A schematic overview of asthma induction in mice. OVA, ovalbumin; Rhy, rhynchophylline; Rhy-SLNs, rhynchophylline-solid lipid nanoparticles
Figure 2. Physicochemical characterization of Rhy-SLNs. a, transmission electron microscopy images of Rhy-SLNs and SLNs. b, the size distribution and zeta potential distribution of Rhy-SLNs. c, the release of Rhy from SLNs. n = 3 in each group. *p < 0.05, **p < 0.01. Rhy, rhynchophylline; Rhy-SLNs, rhynchophylline-solid lipid nanoparticles.
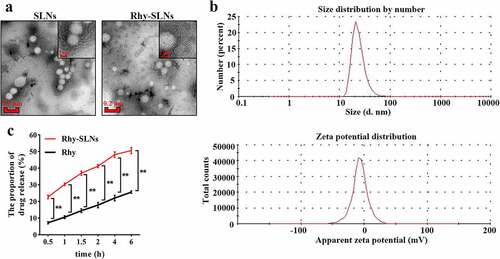
Table 1. Kinetic models applied to estimate obtained results
Figure 3. Rhy-SLNs attenuated the OVA-induced airway inflammation of murine experimental asthma. Mice were subcutaneously injected with 20 μg OVA mixed with 1 mg aluminum hydroxide on days 0, 14, 28, and 42 and administrated aerosolized 1% OVA (w/v) by inhalation from day 21 to day 42. Mice were intraperitoneally injected with 20 mg/kg Rhy-SLNs or 20 mg/kg Rhy at 1 hour before the airway challenge with OVA. a, the count of total inflammatory cells. b-e, the count of neutrophil, eosinophil, lymphocyte, and macrophage. f-i, the concentration of IgE, IL-4, IL-5, and IL-13. J, the representative images of hematoxylin-eosin-stained airway in lung tissues with inflammatory score. n = 6 in each group. *p < 0.05, **p < 0.01. IL, interleukin; OVA, ovalbumin; Rhy, rhynchophylline; Rhy-SLNs, rhynchophylline-solid lipid nanoparticles
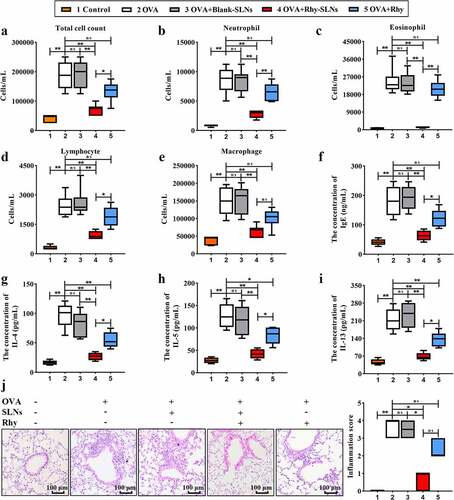
Figure 4. Rhy-SLNs alleviated the OVA-induced oxidative stress of murine experimental asthma. Mice were subcutaneously injected with 20 μg OVA mixed with 1 mg aluminum hydroxide on days 0, 14, 28, and 42 and administrated aerosolized 1% OVA (w/v) by inhalation from day 21 to day 42. Mice were intraperitoneally injected with 20 mg/kg Rhy-SLNs or 20 mg/kg Rhy at one hour before the airway challenge with OVA. a, the representative images of ROS probe-stained airway in lung tissues. b, the mean fluorescence intensity. c and d, the levels of SOD and GSH in lung tissues. n = 6 in each group. *p < 0.05, **p < 0.01. GSH, glutathione; OVA, ovalbumin; Rhy, rhynchophylline; Rhy-SLNs, rhynchophylline-solid lipid nanoparticles; ROS, reactive oxygen species; SOD, superoxide dismutase
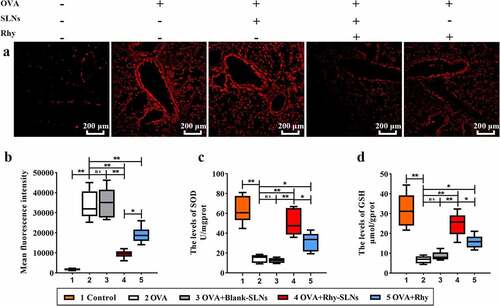
Figure 5. Rhy-SLNs ameliorated the OVA-induced airway remodeling of murine experimental asthma. Mice were subcutaneously injected with 20 μg OVA mixed with 1 mg aluminum hydroxide on days 0, 14, 28, and 42 and administrated aerosolized 1% OVA (w/v) by inhalation from day 21 to day 42. Mice were intraperitoneally injected with 20 mg/kg Rhy-SLNs or 20 mg/kg Rhy at one hour before the airway challenge with OVA. a, the representative images of Masson-stained airway in lung tissues. b and c, the representative images of periodic acid-Schiff-stained goblet cells of airway in lung tissues, which were quantified with the mucus score. Black arrows represented the goblet cells. d, the protein levels of α-SMA and collagen I. n = 6 in each group. *p < 0.05, **p < 0.01. OVA, ovalbumin; Rhy, rhynchophylline; Rhy-SLNs, rhynchophylline-solid lipid nanoparticles; α-SMA, alpha-smooth muscle actin
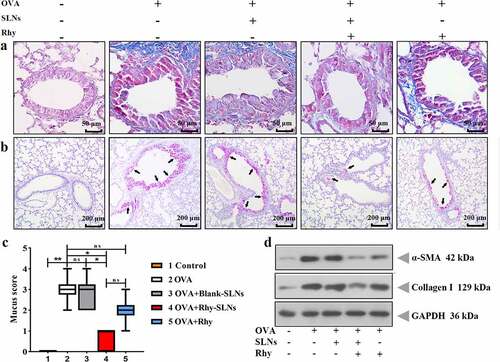
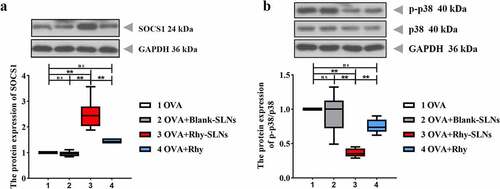
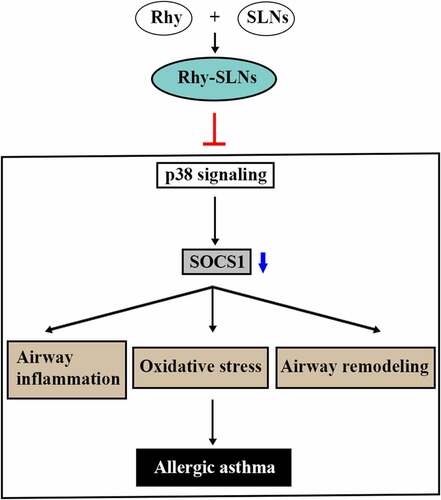
Figure 6. Rhy-SLNs protected airway from OVA-induced damage through the upregulation of SOCS1 by repressing the p38 signaling pathway. Mice were subcutaneously injected with 20 μg OVA mixed with 1 mg aluminum hydroxide on days 0, 14, 28, and 42 and administrated aerosolized 1% OVA (w/v) by inhalation from day 21 to day 42. Mice were intraperitoneally injected with 20 mg/kg Rhy-SLNs or 20 mg/kg Rhy at one hour before the airway challenge with OVA. a and b, western blot bands of SOCS1, p-p38, and p38. n = 6 in each group. *p < 0.05, **p < 0.01. OVA, ovalbumin; Rhy, rhynchophylline; Rhy-SLNs, rhynchophylline-solid lipid nanoparticles; SOCS1, suppressor of cytokine signaling 1