Figures & data
Figure 1. Cellular fates of miRNA and LncRNA maturation. (a) microRNAs, transcribed as primary transcripts from intergenic (pri-miRNA) or, intronic regions (mirtrons, non-canonical). DROSHA-DiGeorge syndrome critical region 8 (DGCR8) complex processes the pri-mRNA structures to produce precursor (pre-mRNA) sequence which is exported from the nucleus to the cytosol for further processing by DICER1 and helicase activity to produce a mature 18–22nts long sequence that undergoes ribonucleoprotein (RNP) assembly with the family of Argonaute proteins (AGO1-4), called as RNA-induced silencing complex (RISC) complex. (b) Long non-coding RNAs are products of Pol II transcription from intergenic regions. They are presumed to undergo processing events including capping, splicing and polyadenylation, in some cases. From left – Natural anti-sense transcripts are transcribed from the opposite(complementary) strands of mRNAs (usually coding in nature). Anti-sense lncRNA and MALAT-1 associated RNA (mascRNA) are produced because of RNAse P processing. U-A-U triple helix at the 3ʹends of mascRNA provides structural stability. sno polyadenylated RNAs (SPA) are 3ʹpolyadenylated and produced from read-through transcripts that are assembled with sno ribonuclear proteins (snoRNPs). In contrast, sno-lncRNAs lack a 5ʹm7G cap and 3ʹ poly (A) tail and are produced from excited introns post splicing events. Finally, the circular intronic RNAs (ciRNAs) are also a product of intron excision post splicing events often produced as an outcome of 3ʹexonuclease activity
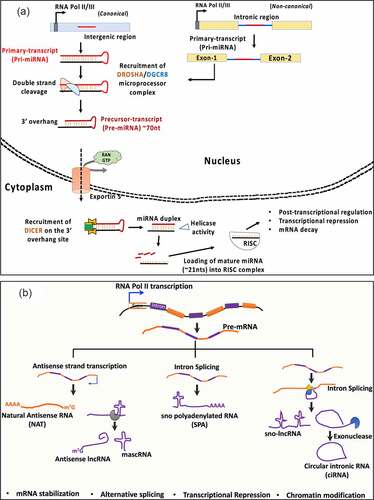
Table 1. List of non-coding RNAs (miRNAs, lncRNAs) detected in the brain regions and cerebrospinal fluid of patients suffering from neurological diseases
Table 2. List of non-coding RNAs (miRNAs, lncRNAs) with their respective genetic targets reported in neurological disorders
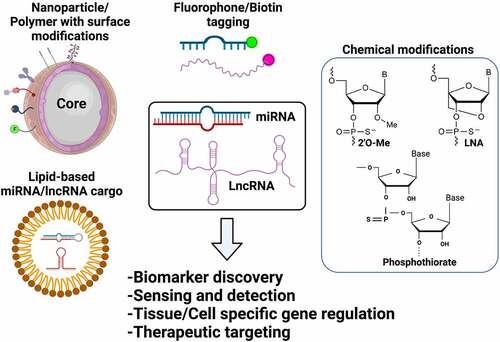
Figure 2. Bioengineering of ncRNAs for biomedical applications. Both miRNAs and lncRNAs can be used for several therapeutic and diagnostic applications based on the bioengineering approaches used that include encapsulation of ncRNAs in functionalized nanoparticles, lipid/polymer based nanocarriers, terminal modifications including biotin or fluorophore tagging or chemical modifications by adding functional groups (i.e., 2ʹO-Methyl, P = S bonds) or by bridging the 2ʹoxygen with 4ʹcarbon as represented in the figure