Figures & data
Figure 1. The expression of circRNA in normal liver cells, HCC cells and DDP resistant HCC cells. (a) HCC cell lines Huh7; (b) HCC cell lines MHCC97L, **P < 0.01.
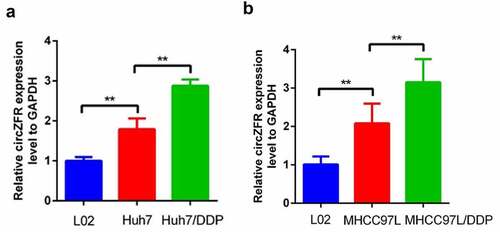
Figure 2. CircZFR promoted DDP resistance in HCC cells. Cell viability (a) and apoptosis (b) of circZFR overexpressed or empty vector transfected HCC cells treated with different concentrations of DDP for 48 h; Cell viability (c) and apoptosis (d) of sh-circZFR or sh-NC transfected DDP resistant HCC cells treated with different concentrations of DDP for 48 h; **P < 0.01.
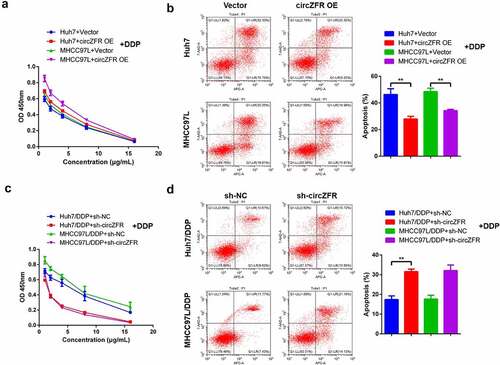
Figure 3. CAFs-derived exosomes might be involved in the regulation of circZFR expression and DDP resistance in HCC cells. (a) The expression of circZFR in CAFs was significantly higher than that in NFs; CAFs significantly increased the cell viability of Huh7 (b) and MHCC97L (c) cells after DDP treatment; (d) CM-DIL labeled exosomes in HCC cells were significantly reduced after GW4869 treatment; (e) and (f) Blocking the secretion of CAFs exosomes significantly increased the apoptosis rate of HCC cells cultured by medium supplemented with CAFs; (g) The blocking of CAFs exosomes secretion significantly decreased the expression level of circZFR in HCC cells cultured by medium supplemented with CAFs; **P < 0.01.
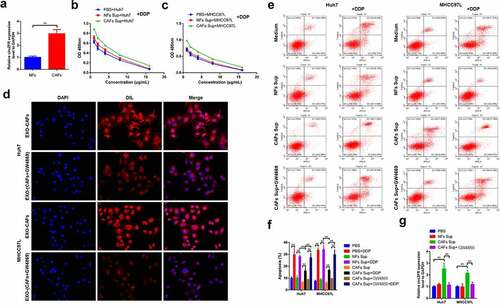
Figure 4. CAFs-derived exosomes deliver circZFR to HCC cells and inhibit the STAT3/NF-κB pathway, promoting DDP resistance of HCC cells. (a) NFs and CAFs-derived exosomes photographed by transmission electron microscopy; (b) The diameter distribution of exosomes ranged around 120 nm; (c) The expressions of CD9, CD63 and CD81 exosome markers in the isolated exosomes were detected by Western blot; (d) CAFs-derived exosomes significantly increased the expression level of circZFR in HCC cells; (e) The phosphorylation levels of STAT3 and NF-κB in HCC cells were significantly decreased by CAFs-derived exosomes culture; (f) CAFs-derived exosomes culture could significantly improve the viability of HCC cells after DDP treatment; **P < 0.01.
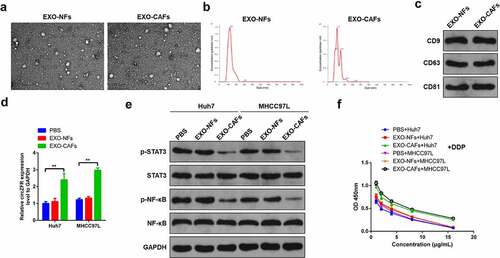
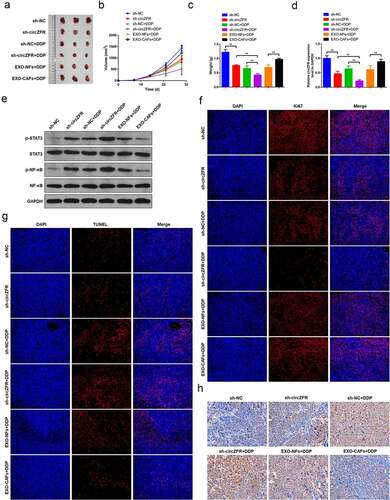
Figure 5. In vivo tumor transplantation confirmed that CAFs-derived exosomes promote DDP resistance of HCC through circZFR. (a) Tumor tissue photos of each group; Tumor tissue volumes (b) and weights (c) in each group; (d) The expression level of circZFR in tumor tissues of each group; (e) Phosphorylation levels of STAT3 and NF-κB in tumor tissues of each group; Ki67 (f) and TUNEL (g) immunofluorescence staining of tumor tissues in each group; H: The levels of cleaved Caspase-3 in tumor tissues of each group detected by immunohistochemistry, **P < 0.01.
Supplemental Material
Download JPEG Image (32.5 KB)Data availability statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
