Figures & data
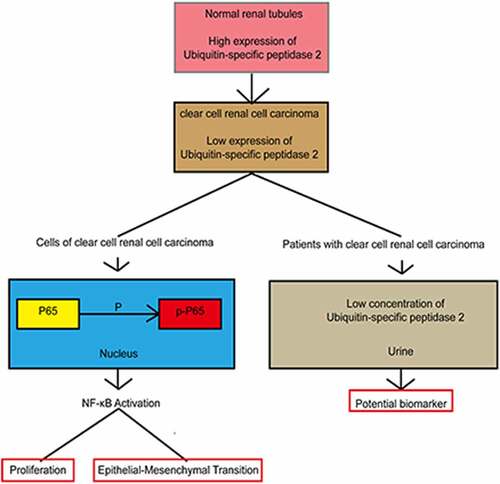
Figure 1. Ubiquitin-specific peptidase 2 downregulation in clear cell renal cell carcinoma is associated with better clinical stage and prognosis. (a) The mRNA-transcriptome sequencing was used to identify mRNAs important in clear cell renal cell carcinoma between tumor tissues and normal tissues. The numbers of highly expressed genes (red) and lowly expressed genes (blue) were shown. (b) Gene Expression Profiling Interactive Analysis showed that the expression of ubiquitin-specific peptidase 2 in paracancerous tissues (gray) was higher than in tumor tissue (red). (c) The expression of ubiquitin-specific peptidase 2 significantly differed between patients with stage I and stage II shown using Gene Expression Profiling Interactive Analysis. (d), (e) Furthermore, Gene Expression Profiling Interactive Analysis revealed that higher expression of ubiquitin-specific peptidase 2 was positively correlated with a better clinical clear cell renal cell carcinoma prognosis including overall survival and disease-free survival. (f) Human Protein Atlas showed that ubiquitin-specific peptidase 2 was mainly enriched in the renal tubules. (g) The Human Protein Atlas showed that ubiquitin-specific peptidase 2 protein expression was higher in the kidney than in other normal tissues. (h) The group that showed a high expression of ubiquitin-specific peptidase 2 tended to have a higher survival probability for Human Protein Atlas. *p < 0.05. Abbreviations: KIRC, kidney renal cell carcinoma; HR, hazard ratio.
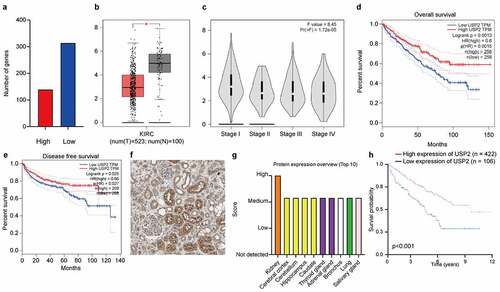
Figure 2. Ubiquitin-specific peptidase 2 knockdown promoted clear cell renal cell carcinoma cell proliferation, migration, and invasion in vitro. (a) The expression of ubiquitin-specific peptidase 2 was low in clear cell renal cell carcinoma tissues detected using quantitative real-time polymerase. (b) The immunohistochemistry results showed that ubiquitin-specific peptidase 2 was richer in renal proximal tubule. (c) The concentrations of ubiquitin-specific peptidase 2 in urine samples of patients with different stages of clear cell renal cell carcinoma were lower than those of healthy volunteers. (d) The concentration of ubiquitin-specific peptidase 2 in urine samples of patients with clear cell renal cell carcinoma was significantly lower than that in healthy volunteers. (e) Protein expression of ubiquitin-specific peptidase 2 in 769-P, 786–0, and Caki-1 cells detected using Western blot. (f) The knockdown efficiency of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma cells shown using quantitative real-time polymerase chain reaction. (g), (h) The results of Cell Counting Kit-8 and clone formation assays showed that ubiquitin-specific peptidase 2 knockdown enhanced the proliferation rate of clear cell renal cell carcinoma cells. (i) Wound healing assays demonstrated that ubiquitin-specific peptidase 2 knockdown promoted greater migration of clear cell renal cell carcinoma cells. Three groups, Si-negative control and Si-ubiquitin-specific peptidase 2–1 for 786–0, Si-ubiquitin-specific peptidase 2–1 for 769-P were healed in 48 hours. (j) Invasion assays revealed that ubiquitin-specific peptidase 2 knockdown promoted the invasive ability of clear cell renal cell carcinoma cells. **p < 0.01, and ***p < 0.001.
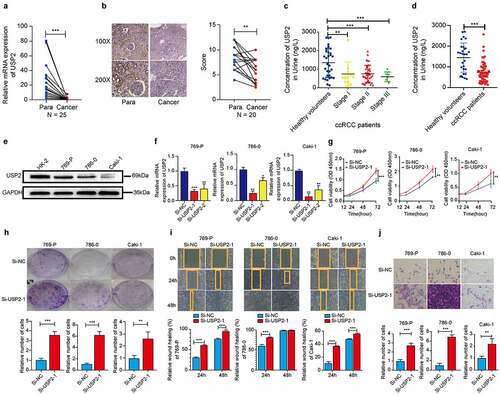
Table 1. The prediction of ubiquitin-specific peptidase 2 between clear cell renal cell carcinoma patients and healthy volunteers.
Figure 3. Ubiquitin-specific peptidase 2 overexpression suppressed clear cell renal cell carcinoma cells proliferation in vitro and in vivo. (a) The over-expression efficiency of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma cells shown using quantitative real-time polymerase chain reaction. (b, c) The results of Cell Counting Kit-8 and clone formation assays showed that ubiquitin-specific peptidase 2 overexpression suppressed the proliferation rate of clear cell renal cell carcinoma cells. (d) Wound healing assays demonstrated that ubiquitin-specific peptidase 2 overexpression inhibited migration ability of clear cell renal cell carcinoma cells. Both Vector and stably overexpressing ubiquitin-specific peptidase 2 groups for 786–0 cells healed at 48 hours. (e) Invasion assays revealed that ubiquitin-specific peptidase 2 overexpression prevented the invasive ability of clear cell renal cell carcinoma cells. (f) The size of tumor was significantly inhibited in the stably overexpressing ubiquitin-specific peptidase 2 vector group than in the lentivirus vector group. (g) Tumors in ubiquitin-specific peptidase 2 overexpression group were relatively lighter in weight. (h) Tumor growth curves for the lentivirus-Vector group and lentivirus-overexpression-ubiquitin-specific peptidase 2 group. Cells in the Vector and overexpression-ubiquitin-specific peptidase 2 groups were transfected with plasmid. The lentivirus-Vector and lentivirus-overexpression- ubiquitin-specific peptidase 2 groups were transduced with lentivirus. **p < 0.01 and, ***p < 0.001.
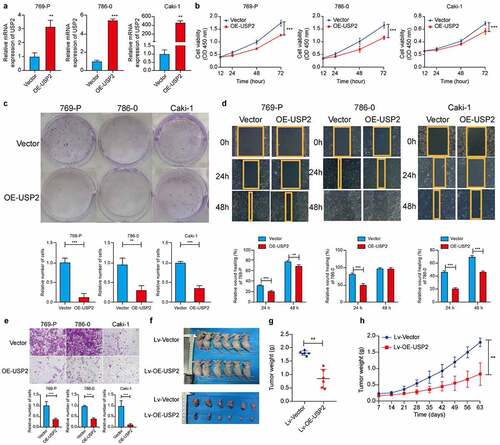
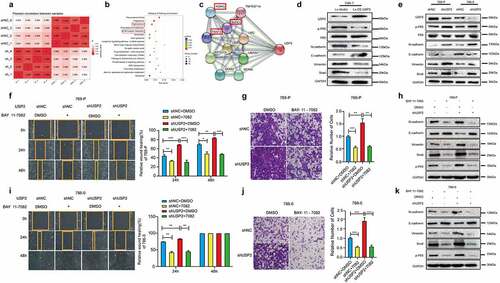
Figure 4. Ubiquitin-specific peptidase 2 inhibited epithelial-mesenchymal transition in clear cell renal cell carcinoma cells by downregulating nuclear factor κB pathway. (a) Pearson correlation between shRNAs for negative control and ubiquitin-specific peptidase 2 in 786–0 cells. (b) Tight junction and extracellular matrix (ECM)-receptor interaction, epithelial-mesenchymal transition-related pathways, were changed remarkably. (c) The results of String showed that ubiquitin-specific peptidase 2 was related to NF-κB pathway gene as inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma (IKBKG), receptor-interacting protein kinase 1 (RIPK1), and TNF receptor associated factors 2(TRAF2). (d) The validation of the lentivirus vector and lentivirus for stably overexpressing ubiquitin-specific peptidase 2 in Caki-1, and the related genes expression for both NF-κB and epithelial-mesenchymal transition pathways. (e) The validation of shRNA negative control and shRNA ubiquitin-specific peptidase 2 in 769-P and 786–0 cells, along with the related genes expression for both NF-κB and epithelial-mesenchymal transition pathways. (f), (g) The migration and invasion abilities of 769-P cells were inhibited by BAY 11–7082 treatment in the shRNA ubiquitin-specific peptidase 2 group and shRNA negative control group. (h) The related gene expression of NF-κB and epithelial-mesenchymal transition pathways are shown, with or without BAY 11–7082. (i), (j) The migration and invasion abilities of 786–0 cells were inhibited by BAY 11–7082 treatment in the shRNA ubiquitin-specific peptidase 2 group and shRNA negative control group. (k) The related protein expression of NF-κB and epithelial-mesenchymal transition pathways are shown, with or without BAY 11–7082. *p < 0.05, **p < 0.01, and ***p < 0.001.