Figures & data
Table 1. The relationship between LRP8 and clinical factors of NSCLC patients
Figure 1. LRP8 is related to the poor prognosis of NSCLC patients. (a–c) Expression of LRP8 in NSCLC tissues as analyzed by TIMER and StarBase3.0 databases. (d) Western blotting assays to detect the expression of LRP8 in seven NSCLC cell lines and the normal bronchial epithelioid cells. (e) Immunohistochemistry staining of two representative cases showing the expression and location of LRP8 in NSCLC tissues. (f) Four-grid table showing the statistical difference of LRP8 level between tumor tissues and normal adjacent tissues. (g) Kaplan–Meier curve based on LRP8 expression in 60 NSCLC patients (log-rank test, p < 0.05). n.s, no significant difference, *p < 0.05, **p < 0.01. At least three independent biological experiments were repeated, and the data were presented as mean ± SD.
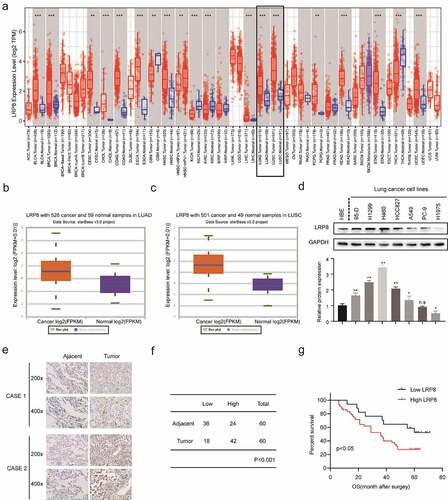
Figure 2. LRP8 promoted NSCLC proliferation in vitro. Western blotting experiments were conducted to validate the transfection efficiency of LRP8 siRNA in H460 and H1299 (a) and LRP8 overexpression plasmid in H1975 (d). CCK-8 assay was used to evaluate the proliferation ability of H460 and H1299 cells transfected with LRP8 siRNAs (b) and H1975 cells with LRP8 plasmid (e). (c) Colony formation analysis showing differences in H1299 and H460 cell proliferation among the three groups. (f) H1975 cell viability was measured using colony formation analysis. **p < 0.01. All experiments were performed independently at least three times, and the results were presented as mean ± SD.
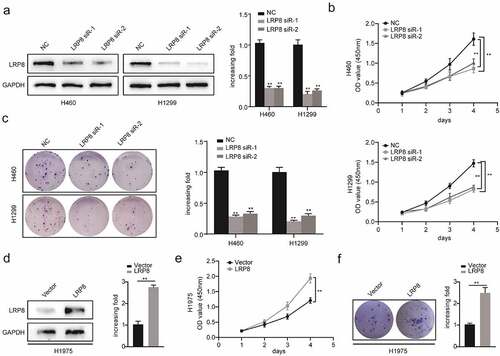
Figure 3. LRP8 enhanced the metastasis of NSCLC cells. (a) Transwell analysis was performed to compare the metastasis potential in the LRP8 knockdown group. (b) H1975 cells migration and invasion capability were detected by transwell assays. The expression of proteins related to metastasis in H1299 and H460 cells (c) and H1975 cells (d) were detected by Western blotting. **p < 0.01. At least three replicate experiments were performed, and the final results were presented as mean ± SD.
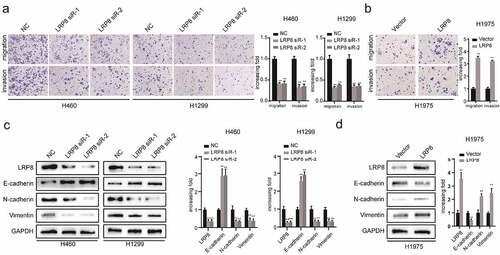
Figure 4. LRP8 motivated NSCLC cells development and metastasis via the Wnt/β-catenin signaling pathway. (a) Expression of Wnt/β-catenin signaling components after silencing LRP8 as detected by Western blotting assays. (b) Expression of Wnt/β-catenin signaling-related factors in overexpression LRP8 group of H1975 cells. CCK-8 assay (c) and colony formation analysis (d) were carried out to evaluate the proliferation abilities of H1299 and H460 cells transfected with LRP8 siRNA or negative vector or LiCl and LRP8 siRNA. (e) Invasion and migration of H1299 and H460 cells after LRP8 downregulation and LiCl addition as detected by Transwell assay. (f) Western blotting analysis for E-cadherin, Vimentin, and N-cadherin to detect the effect of LiCl in LRP8 knockdown. (g) Western blotting assays were performed to elaborate the role of LiCl in Wnt/β-catenin signaling-related factors induced by LRP8 silencing. **p < 0.01. Each experiment was repeated in three independent trials, and mean ± SD was used to describe the results.
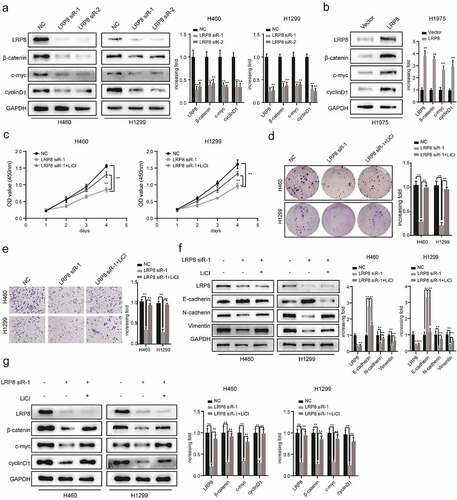
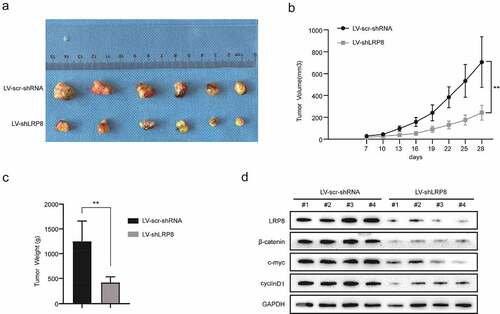
Figure 5. Knockdown of LRP8 inhibited tumor growth in vivo. (a) The pictures of nude mice injected with Lv-sh-LRP8 and corresponding control and the tumors formed after 28-day feeding. (b–c) Tumor volumes and weights were calculated between the two groups. (d) Western blotting experiments detecting the expression of Wnt/β-catenin signaling components and LRP8 expression in subcutaneous tumors. **p < 0.01. Three independent trials in each experiment were needed, and the data were presented as mean ± SD.
Data availability statement
The datasets used or analyzed in this study are available from the corresponding author Ziling Fang upon reasonable request.
