Figures & data
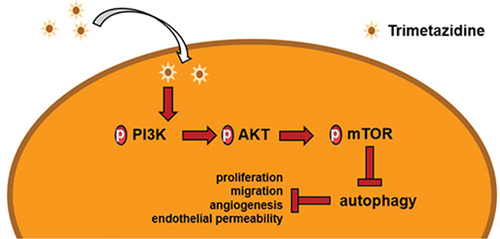
Figure 1. TMZ inhibited the proliferation of HRECs induced by HG.
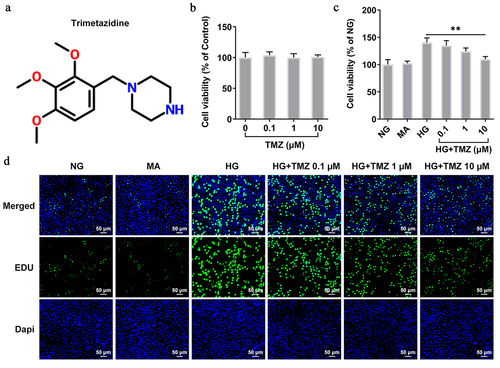
Figure 2. TMZ inhibited migration, invasion, and angiogenesis of HG-induced HRECs.
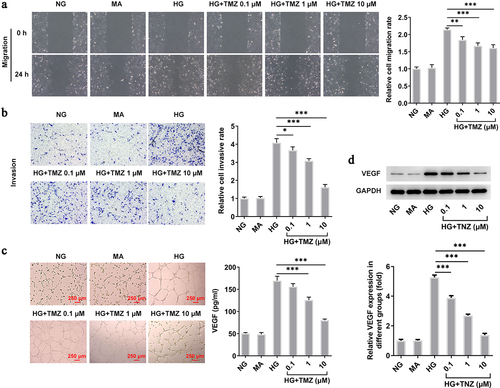
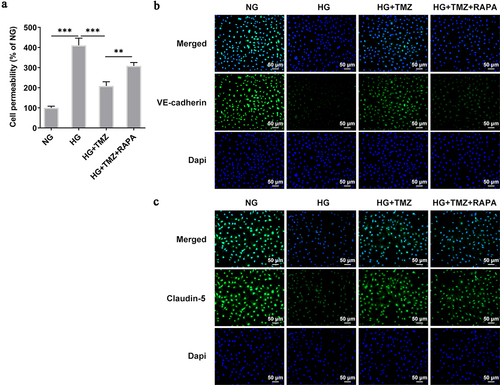
Figure 3. TMZ decreased the permeability of HRECs cells induced by HG, and increased the expression of Claudin-5 and VE-cadherin proteins.
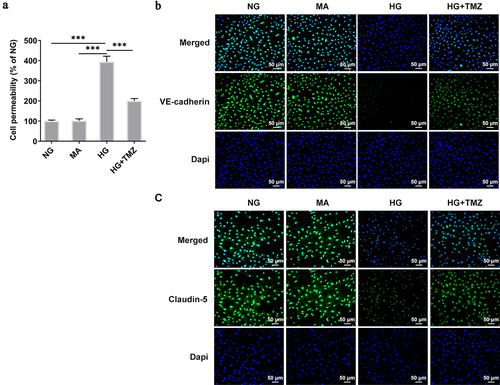
Figure 4. TMZ inhibited autophagy mediated by PI3K/Akt/mTOR pathway.
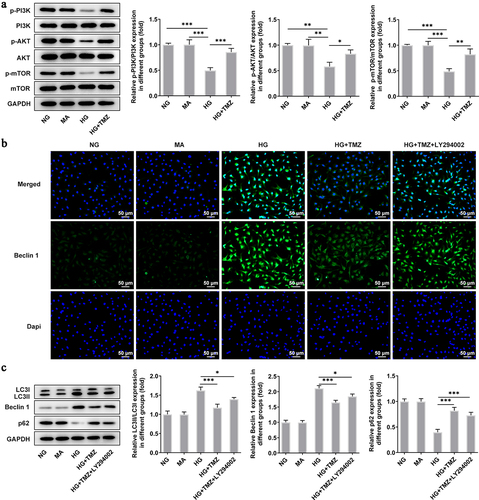
Figure 5. RAPA reversed the inhibitory effect of TMZ on HRECs proliferation, migration, invasion and angiogenesis induced by HG.
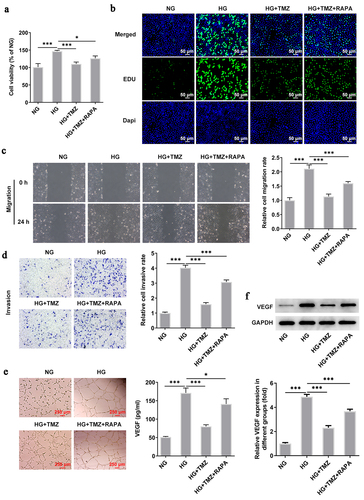
Figure 6. RAPA reversed the inhibitory effect of TMZ on HRECs proliferation, migration, invasion and angiogenesis induced by HG.