Figures & data
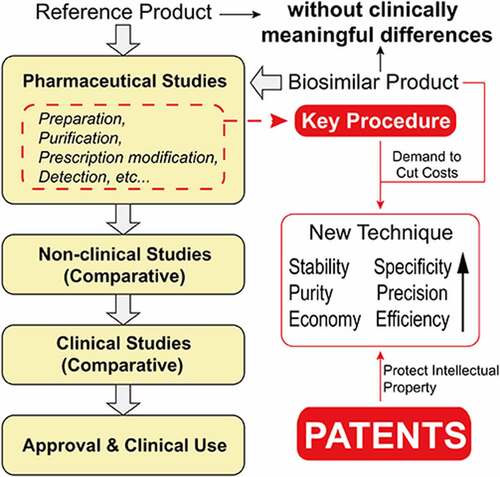
Figure 1. Numbers of the research and development of different biosimilars in China. Adalimumab and bevacizumab are the hotspots in the Chinese biosimilar market, while mAbs with new targets show less competitions.
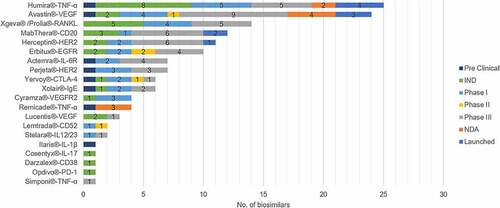
Table 1. Representative companies and the R&D progress of their biosimilar products.
Figure 2. The developing process of the reference and biosimilar products. The elements of their processes are similar, both containing pharmaceutical studies, non-clinical studies (animal studies), and clinical studies. However, biosimilar products pay more attention on comparative studies and their indications can be extrapolated.
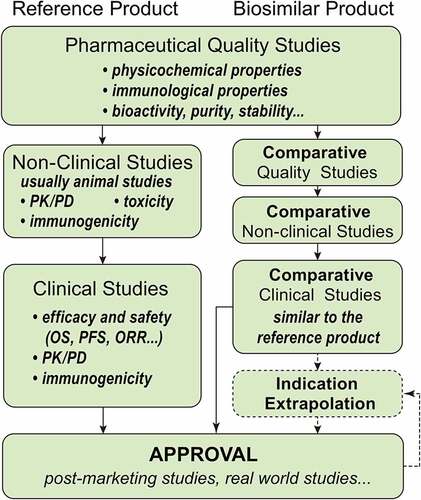
Figure 3. Outline of biosimilar patents in China. a The classification of biosimilar patents and the distribution. b Different targets of biosimilars and the patents of each target. c Trend analysis for Chinese patents on mAb biosimilars.
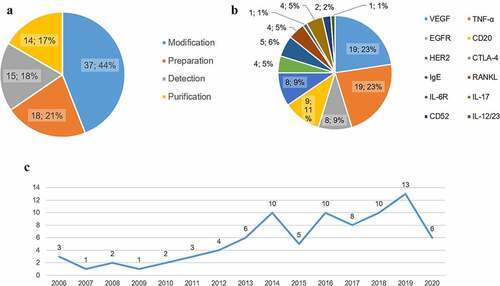
Table 2. The patents of anti-TNF-α antibody biosimilar.
Table 3. The patents of anti-VEGF antibody biosimilar.
Table 4. The patents of anti-CD20 antibody biosimilar.
