Figures & data
Figure 1. Schematic representation of β-catenin protein structure.
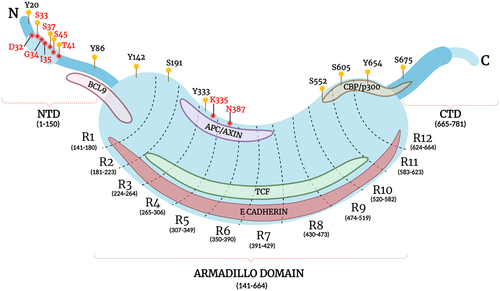
Figure 2. Mechanisms controlling β-catenin activation and dynamic localization.
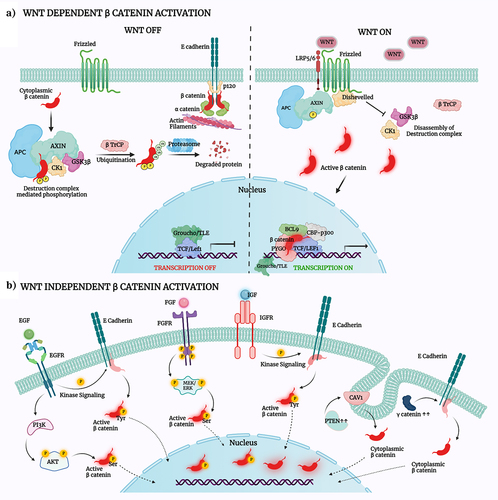
Figure 3. Transcriptional targets of β-catenin and their implications in promoting the hallmarks of cancer.
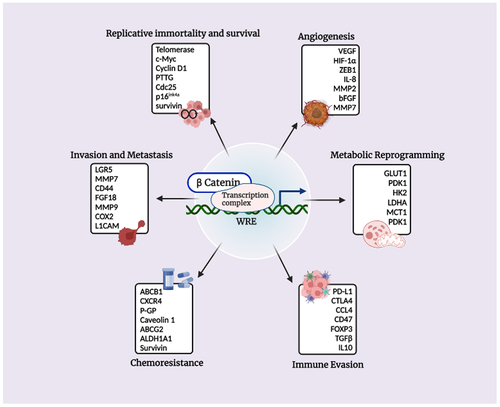
Figure 4. β-catenin enhanceosome complex and therapeutic alternatives.
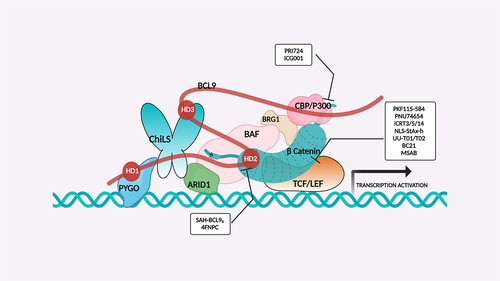
Table 1. A comprehensive list of β-catenin inhibitors with structures and mode of action.
Table 2. A comprehensive list of β-catenin natural inhibitors along with the structure and mode of action.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
