Figures & data
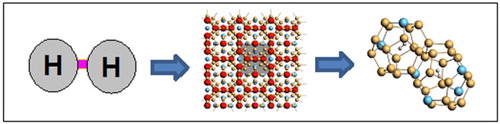
Figure 1. The relative gravimetric performance of physisorption, spillover, and chemisorption (complex hydride) materials towards achieving the minimum material-based targets for on-board reversible fuel storage (from Miller et al. [Citation2]).
![Figure 1. The relative gravimetric performance of physisorption, spillover, and chemisorption (complex hydride) materials towards achieving the minimum material-based targets for on-board reversible fuel storage (from Miller et al. [Citation2]).](/cms/asset/391c90fa-29cf-40a7-a9ea-c781b6ac147d/tmrl_a_1396261_f0001_c.jpg)
Figure 2. (a) Extended crystal structure of the intermetallic clathrates (Type I). Red = volume space for guest atoms such as Ba, Na, and Li. Blue = substitutional framework atoms such as C, Al, and Cu. The unit cell is indicated by grey box. (b) Occupiable volume for H2 exists in the cages of both the 20-atom pentagonal dodecahedron clusters (2a Wyckoff sites) and 24-atom tetrakaidecahedron clusters (6d Wyckoff sites).
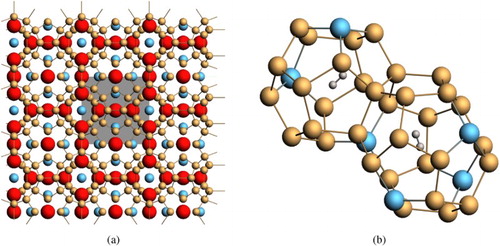
Figure 3. Predicted internal binding energy and capacity for intermetallic clathrates. The range over which binding interactions of H2 is favourable for room temperature storage is noted (a) hydrogenation of Si46, C6Si40, Ba8Al8Si38, and Ba8Cu8Si38 and (b) hydrogenation of Si46, C6Si40, Na8Al8Si38, and Li8Al8Si38.
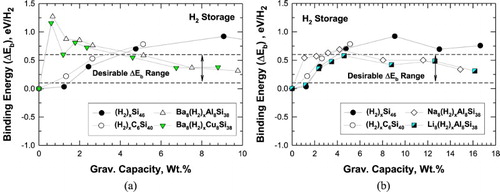
Figure 4. Predicted gravimetric capacity (materials basis) and lattice constant for intermetallic (hybrid) clathrates: (a) hydrogenation of Si46, C6Si40, Ba8Al8Si38 and Ba8Cu8Si38 and (b) hydrogenation of Si46, C6Si40, Na8Al8,Si38, and Li8Al8Si38. Vertical lines mark the system-level targets prescribed by DOE.
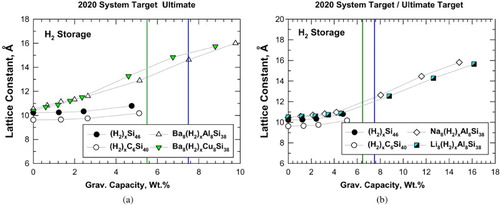
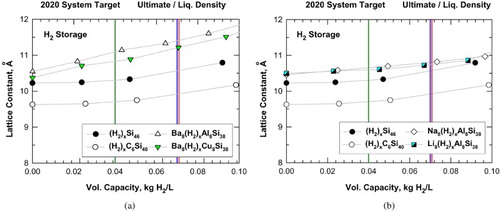
Figure 5. Predicted volumetric capacity and lattice constant for intermetallic clathrates: (a) hydrogenation of Si46, C6Si40, Ba8Al8Si38, and Ba8Cu8Si38 and (b) hydrogenation of Si46, C6Si40, Na8Al8Si38, and Li8Al8Si38. Vertical lines mark the system-level targets prescribed by DOE.