Figures & data
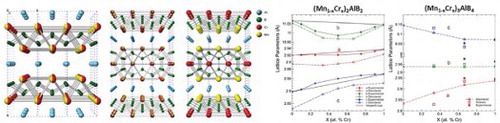
Figure 1. Crystal structures of, a) M2AlB2, b) M3AlB4, c) disordered (Mn1-xCrx)2AlB2, d) disordered (Mn1-xCrx)3AlB4 and e) ordered Mn2CrAlB4. Note that in the 212 structure (a), the layers stack along the b-direction, while in the 314 (b) they stack along the c-direction.
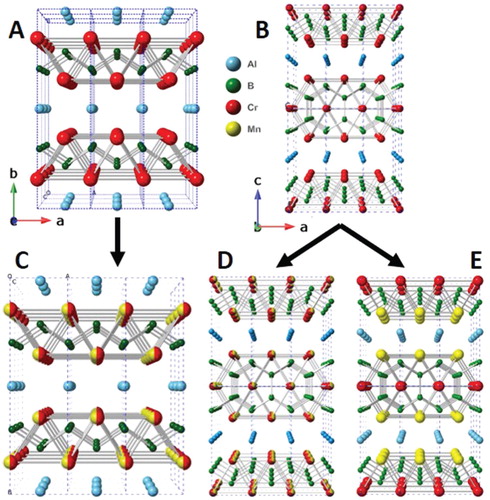
Figure 2. Calculated formation enthalpies, ΔH, at 0 K and Gibbs free energies of formation, ΔG, at T > 0 K for, a) (Mn1-xCrx)2AlB2 and, b) (Mn1-xCrx)3AlB4 solid solutions. In (a), enthalpies of ternaries, viz. −67 and −30 meV/atom for Mn2AlB2 and Cr2AlB2 respectively, are used as reference points (zero).
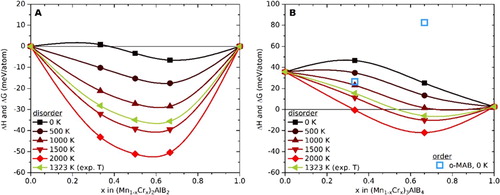
Table 1. Identified equilibrium simplex for (Mn1-xCrx)2AlB2 and (Mn1-xCrx)3AlB4 phases
Table 2. Calculated and experimental LPs and Vuc of (Mn1-xCrx)2AlB2 solid solutions.
Table 3. Experimental and calculated LPs and Vuc for (Mn1-xCrx)3AlB4 with disorder (D) and order (O) of Mn and Cr.
Figure 3. a) XRD patterns of (Mn1-xCrx)2AlB2 sold solutions reacted at 1050°C for 5 h as a function of x. Si powder was added as a reference. b) Lattice parameters, and c) unit cell volumes of (Mn1-xCrx)2AlB2 solid solutions as a function x. Solid lines and symbols denote the experimental LPs; dashed lines and open symbols, denote calculated LPs.
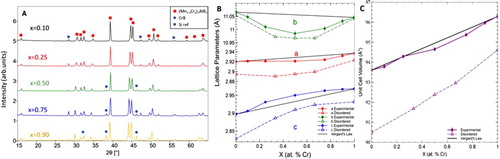
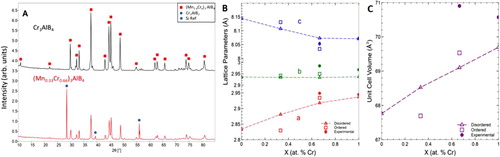
Figure 4. Structural characteristics of (Mn1-xCrx)3AlB4 solid solutions, a) XRD patterns of Cr3AlB4 and (Mn0.33Cr0.66)3AlB4 powders b) Lattice parameters, and c) unit cell volume as a function of Cr substitution. Solid lines and symbols denote experimental LPs; dashed lines and open symbols, denote calculated LPs.