Figures & data
Table 1. Lattice constants, cell volume, and formation energies, , of M2TiAlC2 MAX phases (M = Cr, V, Mo, Ti, Nb, Ta, Hf, Zr, Sc, Y, and La).
Figure 1. The density of states of (a) Ti3AlC2, (b) Zr2TiAlC2, (c) Hf2TiAlC2, (d) Sc2TiAlC2 and (e) Y2TiAlC2.
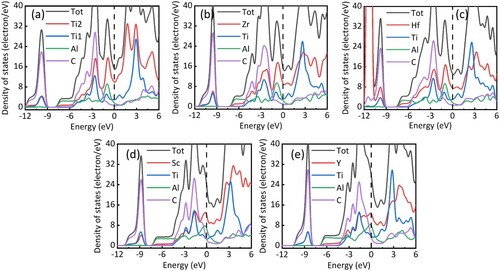
Figure 2. The charge differences of Ti3AlC2 and M2TiAlC2 MAX phases at the same isosurface with isovalue are 0.05 electrons/bohr3.
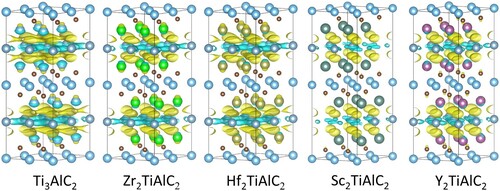
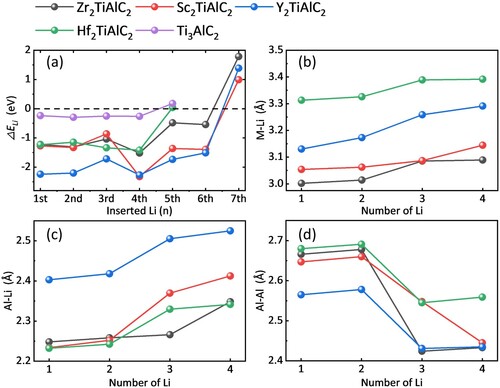
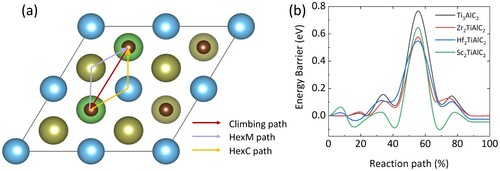
Figure 3. Structure of M2TiAlC2 MAX phases and initial lithium storage sites, where the black dotted line represents different interstices.
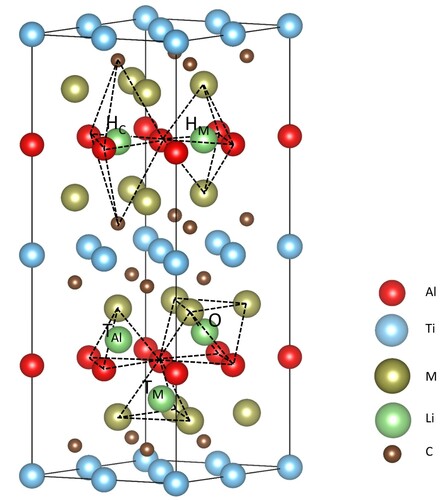
Table 2. Charge transfer with reference to isolated atoms (in electrons; calculated by the Bader approach) for lithiation of M2TiAlC2 MAX phases.