Figures & data
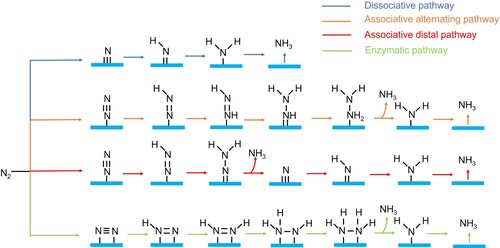
Figure 1. Possible reduction pathways in electrochemical nitrogen conversion, leading to a variety of nitrogen-containing species with different nitrogen oxidation states.
Table 1. Catalytic performances of SACs for ENRR and ENOxRR.
Figure 4. (a) Schematic illustration of synthetic procedure for AuSA/np-MoSe2. (b) NH3 yield rates and (c) FE at different applied potentials. Copyright 2022, John Wiley and Sons [Citation100]. (d) Scheme of the synthetic procedure for Ru SAs/N-C. (e) FE and (f) NH3 yield rates at different applied potentials. Copyright 2018, John Wiley and Sons [Citation99]. (g) FEs over Rh/MnO2 in a dilute electrolyte and water-in-salt electrolytes. Copyright 2022, Elsevier [Citation119].
![Figure 4. (a) Schematic illustration of synthetic procedure for AuSA/np-MoSe2. (b) NH3 yield rates and (c) FE at different applied potentials. Copyright 2022, John Wiley and Sons [Citation100]. (d) Scheme of the synthetic procedure for Ru SAs/N-C. (e) FE and (f) NH3 yield rates at different applied potentials. Copyright 2018, John Wiley and Sons [Citation99]. (g) FEs over Rh/MnO2 in a dilute electrolyte and water-in-salt electrolytes. Copyright 2022, Elsevier [Citation119].](/cms/asset/226206f4-0b71-448e-99d7-bc13be4e03d4/tmrl_a_2209156_f0004_oc.jpg)
Figure 5. (a) Transmission electron microscopy (TEM) image of FeSA-N-C. Scale bar, 50 nm. (b) The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of FeSA-N-C. Scale bar, 2 nm. (c) NH3 yield rates and FE at different applied potentials. (d) Surface-area-normalized NH3 yield rate at different applied potentials on FeSA-N-C and N-C. Copyright 2019, Spring Nature [Citation106]. (e) Schematic illustration of the synthetic process of Cu-N-C. (f) NH3 FE at different applied potentials. (g) i–t curve. Copyright 2022, John Wiley and Sons [Citation44]. (h) NH3 yield rates and (i) FE at various applied potentials. Copyright 2021, Elsevier [Citation129].
![Figure 5. (a) Transmission electron microscopy (TEM) image of FeSA-N-C. Scale bar, 50 nm. (b) The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of FeSA-N-C. Scale bar, 2 nm. (c) NH3 yield rates and FE at different applied potentials. (d) Surface-area-normalized NH3 yield rate at different applied potentials on FeSA-N-C and N-C. Copyright 2019, Spring Nature [Citation106]. (e) Schematic illustration of the synthetic process of Cu-N-C. (f) NH3 FE at different applied potentials. (g) i–t curve. Copyright 2022, John Wiley and Sons [Citation44]. (h) NH3 yield rates and (i) FE at various applied potentials. Copyright 2021, Elsevier [Citation129].](/cms/asset/8c5b31ac-74f8-49b4-b31f-2a664071eb43/tmrl_a_2209156_f0005_oc.jpg)
Figure 6. (a) Free-energy diagrams for ENRR on Mo/BCN through different reaction pathways. Copyright 2022, American Chemical Society [Citation111]. (b) N2-TPD curves of Co single-atom embedded N-doped porous carbon (CSA/NPC). Copyright 2019, Royal Society of Chemistry [Citation116]. (c) Optimized Fe-(O-C2)4 configuration, N2 adsorption at end-on and side-on and corresponding Bader charge distribution of *N2 (yellow and blue represent charge accumulation and depletion, respectively). Copyright 2020, John Wiley and Sons [Citation115]. (d) Pots of charge density difference. The red and dark blue regions represent electron density depletion and accumulation, respectively. Copyright 2022, John Wiley and Sons [Citation42]. (e) Free-energy diagrams for HER over Fe-MoS2 and MoS2. Copyright 2020, John Wiley and Sons [Citation104]. (f) Free-energy diagrams for ENO3−RR on M-MoS2 nanosheets. Copyright 2021, John Wiley and Sons [Citation123]. (g) Structure for copper-porphyrin molecule with eight substituent groups. Copyright 2016, American Chemical Society [Citation140]. (h) Schematic illustration of the catalyst depicted structure. Copyright 2022, American Chemical Society [Citation141].
![Figure 6. (a) Free-energy diagrams for ENRR on Mo/BCN through different reaction pathways. Copyright 2022, American Chemical Society [Citation111]. (b) N2-TPD curves of Co single-atom embedded N-doped porous carbon (CSA/NPC). Copyright 2019, Royal Society of Chemistry [Citation116]. (c) Optimized Fe-(O-C2)4 configuration, N2 adsorption at end-on and side-on and corresponding Bader charge distribution of *N2 (yellow and blue represent charge accumulation and depletion, respectively). Copyright 2020, John Wiley and Sons [Citation115]. (d) Pots of charge density difference. The red and dark blue regions represent electron density depletion and accumulation, respectively. Copyright 2022, John Wiley and Sons [Citation42]. (e) Free-energy diagrams for HER over Fe-MoS2 and MoS2. Copyright 2020, John Wiley and Sons [Citation104]. (f) Free-energy diagrams for ENO3−RR on M-MoS2 nanosheets. Copyright 2021, John Wiley and Sons [Citation123]. (g) Structure for copper-porphyrin molecule with eight substituent groups. Copyright 2016, American Chemical Society [Citation140]. (h) Schematic illustration of the catalyst depicted structure. Copyright 2022, American Chemical Society [Citation141].](/cms/asset/73448a83-18ee-44c3-87eb-b91df61e8e76/tmrl_a_2209156_f0006_oc.jpg)
Figure 7. (a) Schematic illustration of ENRR for Cu-Nx structure. (b) Free-energy diagrams for the ENRR on Cu-N2 through different reaction pathways. Copyright 2019, American Chemical Society [Citation117]. Calculated free energies for (c) NO3− adsorption and (d) NO2− adsorption on Cu (111), Cu-N4, and Cu-N2 surfaces, respectively. The brown, grey, blue and red balls represent C, N, Cu and O atoms, respectively. Copyright 2020, John Wiley and Sons [Citation149].
![Figure 7. (a) Schematic illustration of ENRR for Cu-Nx structure. (b) Free-energy diagrams for the ENRR on Cu-N2 through different reaction pathways. Copyright 2019, American Chemical Society [Citation117]. Calculated free energies for (c) NO3− adsorption and (d) NO2− adsorption on Cu (111), Cu-N4, and Cu-N2 surfaces, respectively. The brown, grey, blue and red balls represent C, N, Cu and O atoms, respectively. Copyright 2020, John Wiley and Sons [Citation149].](/cms/asset/d388ed07-7866-4995-9e71-74822dfb38b6/tmrl_a_2209156_f0007_oc.jpg)